franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Hypothetical BoroFluoramine
Creation of more powerful explosives continues to be more of a wishful dream
than reality. The same chemistry employed a century ago remains a mainstay
of the handful of militarily useful compounds, and has long since reached the
practical limit of theoretical caloric values. Can another 20 % of performance
be found ? I'm sure that it's not worth burning the candle to look for it. If you
want the highest attainable energy you are very much now limited to reactions
involving metals with oxygen or flourine. Combining these elements to form
safe metastable compounds that will detonate is the matter at hand.
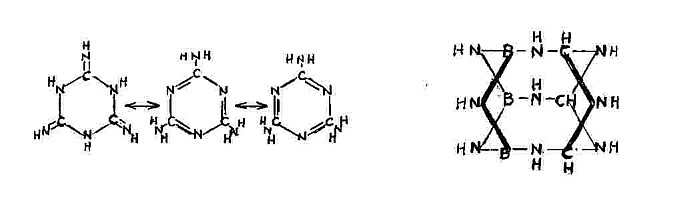
It occurs to me that the 3 Hydrogen Boron bonds of this material Borazine
seen here below _ http://en.wikipedia.org/wiki/Borazine

can combine with 3 imido Nitrogen Carbon bonds of Melamine
seen here below _
to form the condensed caged molecule seen below here on the right.
The Nitrogen imido of Melamine on the right side links to the Boron of Borazine
on the left , and the Hydrogen on the Boron of Borazine on the left side should
attach to the carbon of Melamine on the right. Well maybe.
The next step would be to fluorinate and subplant all of the Hydrogens of the
9 joining amines and the 3 of the carbon to form the desired product.
B3 C3 F12 N9 -> 3BF3 + 3FCN + 3N2
A related thread not well received is here _
http://www.sciencemadness.org/talk/viewthread.php?tid=6070#p...
[Edited on 11-9-2006 by franklyn]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
http://www.sciencemadness.org/talk/viewthread.php?tid=6070#p...
.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Why would they so join? What you've done, in effect, is taken two benzenes and converted them to cross-linked cyclohexanes, a higher energy state.
Something would have to push the propossed reaction, I just don't see it occuring on its own.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Franklyn, I think you read from that Wikipedia entry that borazine does not behave fully aromatically. From the two examples of addition of HCl and
hydride on the "aromatic" ring you probably concluded that just about anything can add on that ring including barely nucleophilic compounds like
melamine.
What you did not consider (beside the lack of nuclophilicity of melamine) is that it is inconvenient for the product to reside in a higher energy
state when there are no obstacles for it to go to a lower energy state. In short, the hypothetical product of the reaction between melamine and
borazine would be a flat polymer. And besides the interaction would be different as you depicted it.
Though in reality no such product could be stabile since, as Wiki says: "Borazines interact with nucleophilic attack at boron and electrophilic attack
at nitrogen." Which means that both the attacking electrophile and nucleophile must be more reactive than the formed electrophilic and nucleophilic
centers in the product (on nitrogen and boron respectively). So, HCl and hydride can add on borazine, melamine can't.
Franklyn, you reason linearly like if you would be solving a mathematical problem, but in chemistry such reasoning always leads to wrong conclusion. I
see many beginners trying to apply the same logic, but it simply does not work and it can get very frustrating if one does not realize this soon
enough. In chemistry you simply can not generalize from a single fact to a single conclusion. You need to use arrays of facts to get out a single
conclusion. In my experience I found that mathematical thinking is the exact opposite of chemical thinking (though I know the comparison is kind of
inappropriate since mathematics is not a science but a separate knowledge).
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
My inspiration is the higher caged Boranes. True just because a bond can be
imagined, does not necessarily mean it can be realized. I tend to apply Occam's
razor liberally but one is constrained by the available reaction pathways of
which I have scant knowledge. Chemistry reviewed in this forum is monotonously
performed in ambient conditons at S.T.P. this betrays the focus of thought with
regard to reactions that are not immediately apparent.
| Quote: | Originally posted by not_important
Why would they so join?Something would have to push the propossed reaction,
I just don't see it occuring on its own. |
I only supposed that bonding might be induced and that some cooking
would be
required, as you emphatically state these two cyclic forms are happy as they are.
| Quote: | Originally posted by Nicodem
the reaction between melamine and borazine would be a flat polymer |
This was my concern since changing the
established ring shape is much harder
than building entirely new shapes from simpler precursers. Also Boron is only known
to bond with two separate Nitrogens the third bond is always hydrgen or carbon.
But would the same barriers at room temperature and one atmosphere still hold
true in a reaction vessel at hundreds of atmospheres ? I recall having read of
compounded melamine boron fire retardant laminates made in this way, though
these too are polymeric forms.
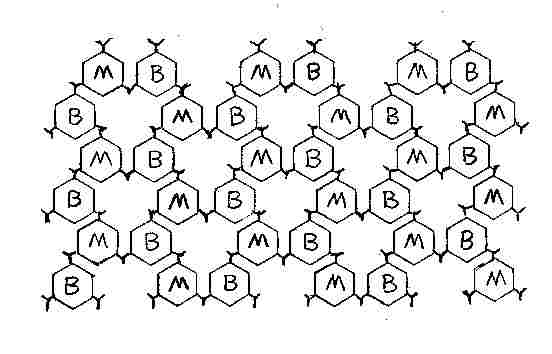
For those of you who favor tiling on the plane I give you
the polymer form below here _
| Quote: | Originally posted by Nicodem
both the attacking electrophile and nucleophile must be more reactive than the formed electrophilic and nucleophilic centers in the product (on
nitrogen and boron respectively). |
I'm not giving up so easily , what if Melamine were Fluorinated before reacting with Borazine. Just a thought.
[Edited on 3-9-2006 by franklyn]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by franklyn
... Also Boron is only known
to bond with two separate Nitrogens the third bond is always hydrgen or carbon.
But would the same barriers at room temperature and one atmosphere still hold
true in a reaction vessel at hundreds of atmospheres ? I recall having read of
compounded melamine boron fire retardant laminates made in this way, though
these too are polymeric forms.
. |
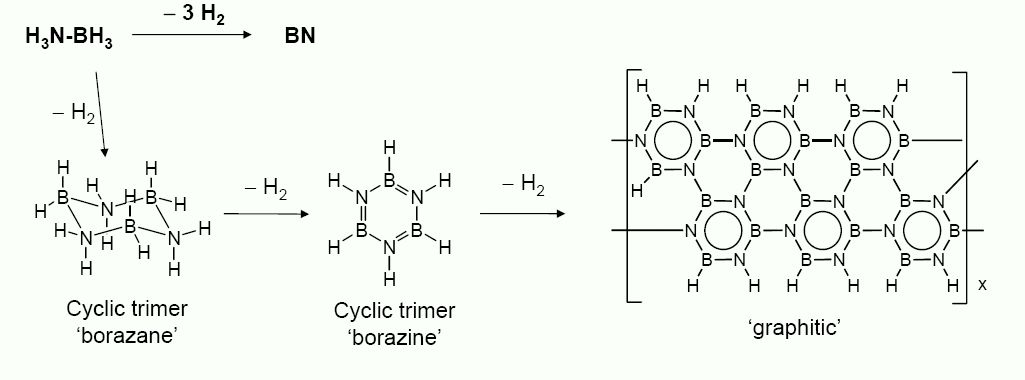
Actually, if you strongly heat a mixture of borax and ammonium chloride, the cool and wash with water, you will find that you have some http://www.physik.unizh.ch/groups/grouposterwalder/kspace/BN...
really high pressure results in the cubic, diamond structured form.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by franklyn
Also Boron is only known to bond with two separate Nitrogens the third bond is always hydrgen or carbon. |
Amend that to short of BN ceramic or Borazon.
[Edited on 3-9-2006 by franklyn]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Or cubic BN, where the nearest neighbors of each boron are 4 nitrogens, and VV
http://www.mext.go.jp/english/news/2000/04/s000406.htm
I don't think your proposed compound would form under pressure, it just doesn't look like it would give maximum density; apply pressure would favour
other compounds.
|
|
|