| Pages:
1
2 |
experimenter_
Harmless

Posts: 38
Registered: 18-2-2016
Member Is Offline
Mood: No Mood
|
|
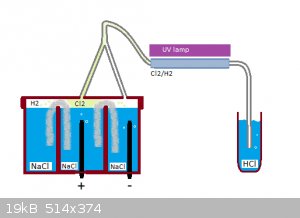
Hydroclhloric acid (and HCl gas) production from salt (NaCl) electrolysis (small scale Chloralkali process)
In brief, a concentrated sea salt solution is electrolysed, the gases (H2/Cl2) are reacted together under UV light and the resulting hydrogen chloride
is dissolved in water.
Previous failed attempts and background ideas can be found in this thread [ https://www.sciencemadness.org/whisper/viewthread.php?tid=21... ]. I think that many of the problems have been solved and this method shows
potential to produce practical amounts of hydroclhloric acid; at least my device. In the current stage of development the key elements of the design
are the following:
- Two graphite electrodes (acquired from zinc batteries)
- A salt water container with two seperate compartments connected electrically with a "salt bridge" made out of filter paper
- A UV black light lamp in parallel with a glass tube where the gases combine
Check the attached schematics for the detailed info. There is also a picture of the device (not very pretty on the outside but she has hidden graces
:-) )
My frist measurement on the efficiency of the device was the following:
A 0.2A input electrode current for 2h produced the equivalent of ~ 0.8ml 10% HCl solution (or 0.08gr HCl or ~ 0.0022 mol HCl). The theoretical amount
according to Faraday law should be 0.015mol. Thus the efficiency is 6.8%.
Of course I'm not convinced that this number is the best efficiency I can get out of the device. For example I detect Cl2 in the output which means
that a bigger UV tube is needed. Or my electronics to light the UV tube do not produce much current (I don't have ballast but a small HV transformer).
Also I see the reaction tube to be full of droplets. Those are made of concentrated HCl that captures air moisture and never reaches the output water.
If the glass tube is opened a white mist (HCl) evolves out of it (it gets hot also). Finally, in the electrolysis chamber, the Cl2 gas has a chance to
touch the surface of the NaOH solution even if it is not bubbled through it (design flaw). Maybe it gets absorbed there? Anyway, there is a lot of
room to apply fixes for better efficiency but even if it stays below 10% that is ok.
About the output capacity, in this crude form at 0.2A input current, the device would give 10ml of azeotropic HCl in 2 days. But the salt bridge can
carry up to 2A and the electrodes can easily acquire more surface. So a target output capacity of 100ml per 2 days is not out of reach. The main
limiting factor is the UV lamp, right now it is of a such small intensity that it can barely handle the gases produced by the 0.2 A electrolysis
current.
In conclusion:
Pros
- Production of HCl out of salt and electricity only
- Production of hydrogen chloride gas. Instead of diluting it in water, other uses might be employed too.
- Production of NaOH out of electrolysis of salt
- Production of Cl2 or H2 separately is possible
Cons
- HCl acid produced is contaminated with dissolved Cl2 (major problem; working on its solution)
- Inherent dangers of explosion. For example, the glass tube where Cl2/H2 gases mix, if not exposed continualy to UV light, can build up a lot of
Cl2/H2 gas that might explode and shatter it (unfortunately plastic tube blocks UV light). Also there is a lot of air space in the electrolysis
container where Cl2/H2 gases could mix. Those are design flaws hopefully to be corrected in later versions. Some "watchdog" electronics could help
with this problem too.
- Cl2 gas is poisonous if escapes in the air (well it is not so much since the smell threshold is far below the dangerous concentrations but still one
cannot leave the device running unattended inside closed space)
- NaOH produced is contaminated with a lot of NaCl (but it is not a big problem for many applications)
Some questions:
- For how long will the device be able to work until some kind of failure happens? For example, will the carbon electrodes corrode too soon. Will the
catholyte mix with the anolyte after some days (and the Cl2 will touch the NaOH)
- What is the maximum concentration of NaOH that can be achieved before the solution in the cathode must be changed with fresh NaCl? Why not reaching
up to 100% ?
- Will a longer UV tube be able to eliminate all of the Cl2 before it touches the water? Should more H2 be added?
[Edited on 10-4-2016 by experimenter_]
|
|
|
macckone
Dispenser of practical lab wisdom
    
Posts: 2168
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
Good writeup
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Question: What is the purpose of the cell of NaCl to the leftmost side of the apparatus? It has no electrode, and I don't think it'll serve as a
reservoir for NaCl, considering how slow it would come over the filter paper. (Edit: Is this a concentric-ring design? That would explain it.)
To answer some of your questions...
1) Hard to say. Usually, the limiting factor is either the electrodes corroding to pieces or the filter paper that is exposed to air drying out (at
least, it was for me). A quick note: You don't need carbon electrodes on both sides. Use an iron or steel cathode; it'll have less internal resistance
and thus transmit more current (for faster yield).
2) Again, hard to say. Theoretical maximum would be a saturated solution of NaOH in water. There shouldn't be much preventing this, because of all the
ionic (and therefore conductive) species in solution.
3) If I recall, electrolysis that splits the water will produce 2 mol of H2 for every mol O2, and electrolysis that splits the salt will produce 1/2
mol of Cl2 at the same rate. At a ratio of 2 mol H2 to 0.5 mol Cl2... no, you shouldn't need to add more hydrogen. More UV light will most likely add
to the yield, but I'm not sure.
Electrolysis, despite being one of my favorite things to do, is not my strong suit in terms of analysis. So if anything above is wrong... well, I
guess you'll just have to take things with a grain of NaCl.
[Edited on 4-10-2016 by elementcollector1]
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
macckone
Dispenser of practical lab wisdom
    
Posts: 2168
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
Limiting factors are filter paper degradation by sodium hydroxide, flow of sodium hydroxide back across the cell and electrode degradation.
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
I think that graphite will be fine for an anode, so long as the amount of chloride ion is kept high, and the anolyte pH is kept fairly neutral or
lower. Also, keep the cell running cool. These things help ensure that you're producing mainly chlorine instead of oxygen. Once you start getting
oxygen at the anode, the oxygen goes ommm nom nom on the graphite, and it starts disintegrating.
|
|
|
experimenter_
Harmless

Posts: 38
Registered: 18-2-2016
Member Is Offline
Mood: No Mood
|
|
Thanks for the interest guys.
Yes, there is one more smaller pvc pipe inside the container glued to the bottom. The cables reach the electrodes through the bottom of the cylindric
pvc box, not visible in the picture.
Quote: Originally posted by elementcollector1  |
3) If I recall, electrolysis that splits the water will produce 2 mol of H2 for every mol O2, and electrolysis that splits the salt will produce 1/2
mol of Cl2 at the same rate. At a ratio of 2 mol H2 to 0.5 mol Cl2... no, you shouldn't need to add more hydrogen. More UV light will most likely add
to the yield, but I'm not sure. |
I think that H2 and Cl2 are produced in equal moles. Na+ and Cl- require only one electron to reduce/oxidize so...
Wiki ( https://en.wikipedia.org/wiki/Chloralkali_process ) says: 2NaCl + 2H2O → Cl2 + H2 + 2NaOH
 I miss scientific humour! I miss scientific humour!
I've been looking around the forum and found some other relevant threads:
https://www.sciencemadness.org/whisper/viewthread.php?tid=22...
https://www.sciencemadness.org/whisper/viewthread.php?tid=25...
https://www.sciencemadness.org/whisper/viewthread.php?tid=17...
Also a link outside the forum:
http://woelen.homescience.net/science/chem/exps/miniature_ch...
(woelen is the owner of this forum?)
It seems that an unglazed flower pot can be used instead of the filter paper bridges and a titanium MMO anode (what is that MMO?) instead of the
carbon electrodes. So, in case the paper bridge is eaten up or the electrodes corrode there are some possible future solutions.
My priority consideration now is to find a practical way to eliminate Cl2 from the output. Even when the H2/Cl2 flow through the UV tube is very slow
the gas don't seem to react completely. I'm afraid that too much excess H2 will be needed or impractically long UV tube in order to eliminate Cl2.
Leaving it in the sun few days will not do the trick.
Some guy suggested to bubble SO2 in the output water. Supposeddly reaction with Cl2 will give H2SO4 + HCl and then we distill the clean HCl out of
H2SO4. But I doubt that SO2 + Cl2 (+ H2O) gives H2SO4 only.
Someone else suggested it is easier to make H2SO4 from the SO2 and then distill HCl out of NaCl. Hm, maybe .. 
[Edited on 11-4-2016 by experimenter_]
|
|
|
experimenter_
Harmless

Posts: 38
Registered: 18-2-2016
Member Is Offline
Mood: No Mood
|
|
New observations
A new run for 9hours at 0.2A was completed. It produced the equivalent of ~2.7ml 10% HCl. The efficiency this time was about
11%. 
An inspection on the insides showed that the electrodes show no sign of corrosion (probably due to the very small current; only 0.2A) and the anolyte
is still acidic. The catholyte is very basic (NaOH) but it has almost dissolved the filter paper that was hanging on this side of the cell.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
| Quote: | | Someone else suggested it is easier to make H2SO4 from the SO2 and then distill HCl out of NaCl. Hm, maybe. |
Jeez, am I missing something here . . . ?
Just to be clear, you can simply toss some salt into a flask, add enough conc. H2SO4 to cover it (reaction is immediate and
vigorous) and dissolve the evolved HCl gas in water using an inverted funnel, et voilà!
When the initial reaction slackens, heating the sol. will drive it to completion!
And using distilled water gives pure acid as only HCl comes over . . .
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Quote: Originally posted by experimenter_  | A new run for 9hours at 0.2A was completed. It produced the equivalent of ~2.7ml 10% HCl. The efficiency this time was about
11%.  |
Very nice experiment, and glad to see that you're improving the process.
Keep going !
Edit:
I read quite a while back an experiment that used a thin terracotta plant pot for the 'membrane' or 'salt bridge'.
Cannot remember where.
[Edited on 11-4-2016 by aga]
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Yeah, the big problem with HCl from salt/acid is that it can take as much as a full second to produce twice that amount of HCl.
|
|
|
experimenter_
Harmless

Posts: 38
Registered: 18-2-2016
Member Is Offline
Mood: No Mood
|
|
To hissingnoise:
| Quote: | | Quote: | | Someone else suggested it is easier to make H2SO4 from the SO2 and then distill HCl out of NaCl. Hm, maybe. |
Jeez, am I missing something here . . . ? |
You are right, but since the forum is called sciencemadness I hope it can host the method of this thread too. It is not the best or
the most efficient but I find it a more elegant way to produce HCl. Something like an art project.
When completed, some other differences compared to the salt/acid method will be:
- If you live by the sea, there is no need to buy anything from anyone
- Besides HCl, it could give H2, Cl2 and NaOH out of nothing
- It is smaller than a distillation setup and more portable (no need for heating mantle, condenser etc..)
The drawback is that it requires patience but consider it like having a salt eating pet that keeps some company.
Don't judge it too soon, the current can rise up to 2A within the same device..
To aga:
| Quote: | Very nice experiment, and glad to see that you're improving the process.
Keep going !
Edit:
I read quite a while back an experiment that used a thin terracotta plant pot for the 'membrane' or 'salt bridge'.
Cannot remember where. |
Thanks aga, I'll keep you updated! I think this is the thread you are talking about (or sth similar):
https://www.sciencemadness.org/whisper/viewthread.php?tid=27...
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
| Quote: | | It is smaller than a distillation setup and more portable (no need for heating mantle, condenser etc.. |
WTF! This is about simply generating and dissolving a soluble gas in water!
Two friggin' containers connected by a short plastic tube . . .
I don't know where you got the idea that distillation was involved!
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
I've done the "flower pot cell" before as an experiment to produce NaOH. It worked, albeit very slowly. The real problem I found is how to permanently
seal the hole in the bottom of the flower pot. Tape falls off and caulk quickly degrades in the basic environment!
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
Quote: Originally posted by experimenter_  |
An inspection on the insides showed that the electrodes show no sign of corrosion (probably due to the very small current; only 0.2A) and the anolyte
is still acidic. The catholyte is very basic (NaOH) but it has almost dissolved the filter paper that was hanging on this side of the cell.
|
One idea is to have a third (small) compartment in between the cathode and anode compartments. This is where fresh NaCl solution is added. The
addition rate should be fast enough to keep the anolyte and catholyte from infiltrating the addition compartment, but not so fast that electrolysis
can't keep the solutions from becoming diluted excessively. The overflow from both compartments can be collected as products.
The separators could be made from bundles of capillary tubing, or something that will allow laminar flow from the addition compartment, while limiting
turbulence that could promote back-mixing between adjacent compartments, if that makes sense.
It might even be possible to use cotton wool as a separator in a larger glass tube, so long as the fresh NaCl flows through it at a rate that keeps
the concentrated products away from it.
|
|
|
experimenter_
Harmless

Posts: 38
Registered: 18-2-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by WGTR  |
One idea is to have a third (small) compartment in between the cathode and anode compartments. This is where fresh NaCl solution is added. The
addition rate should be fast enough to keep the anolyte and catholyte from infiltrating the addition compartment, but not so fast that electrolysis
can't keep the solutions from becoming diluted excessively. The overflow from both compartments can be collected as products.
The separators could be made from bundles of capillary tubing, or something that will allow laminar flow from the addition compartment, while limiting
turbulence that could promote back-mixing between adjacent compartments, if that makes sense.
|
Thanks for the input; I think the idea is a bit advanced for the time being. Adding salt solution and
removing products while electrolysis is running seems too much for me to apply.
But I'll keep your advice on capillary tubing. I could use them as a salt bridge after giving them U shape in case i can't find any other material
that can withstand the lye solution. I would like to avoid the "flower pot" method.
Quote: Originally posted by MrHomeScientist  | | I've done the "flower pot cell" before as an experiment to produce NaOH. It worked, albeit very slowly. The real problem I found is how to permanently
seal the hole in the bottom of the flower pot. Tape falls off and caulk quickly degrades in the basic environment! |
I use "Bison montage kit" ( https://www.bison.nl/static/uploads/bison/folders/montagekit... ). It can withstand Cl2 and NaOH. I'm sure it can adhere onto the flower pot too.
If you can find this glue, it worths to give it a try.
|
|
|
experimenter_
Harmless

Posts: 38
Registered: 18-2-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by hissingnoise  | | Quote: | | It is smaller than a distillation setup and more portable (no need for heating mantle, condenser etc.. |
WTF! This is about simply generating and dissolving a soluble gas in water!
Two friggin' containers connected by a short plastic tube . . .
I don't know where you got the idea that distillation was involved!
|
I wasn't aware that this method you describe could be employed practically; I was thinking that one should
distill hydrochloric acid out of a dilute solution of H2SO4 and sea salt so that there is some water to come over together with the HCl gas in order
to capture it (similar way to making nitric acid).
So, if a way to regain sulfuric acid from the sodium bisulfate could be found, this method could be compared with the direct NaCl electrolysis
described in the thread. For example, if electrolysis of sodium bisulfate (towards sulfuric acid and NaOH) in a membrane cell could be applied, this
would mean that salt can be converted to HCl by the use electricity only. The toxic Cl2 gas, danger of explosion and Cl2 contamination in the product
would be avoided completely in that way but I'm afraid it has some of its own drawbacks too. If recycling the H2SO4 is possible I would consider it.
|
|
|
experimenter_
Harmless

Posts: 38
Registered: 18-2-2016
Member Is Offline
Mood: No Mood
|
|
Another way to remove dissolved Cl2 in the output HCl solution could be to react it with H2O2. In theory, this reaction happens:
HClO + H2O2 --> HCl + H2O + O2
I don't know yet what might happen in practice.
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
Experimenter_, nice to read this thread on your efforts.
I'd like to point out that the efficiency in your first experiment was actually 14.9%, as 0.0022/0.015 = 0.149.
hissingnoise's method is much easier, but requires an acid to start with. Also, you would need to use a long tube from the funnel to your collecting
flask, in order to catch any aerosols.
In you electrolysis setup, you might consider the following:
* Use UV LEDs (check ebay or suppliers whether the required wavelength is available). If you would use a mirroring tube in which you bore holes to fit
your LEDs, you'd have a considerable improvement in efficiency with respect to UV. Your reaction tube runs inside the mirroring UV tube.
* Suspend the UV reaction tube vertically, so that condensation droplets will run into your flask.
* Use the membrane of a lead-acid battery. You'll need the old, wet type of battery. I used the membranes from these batteries with great success
electrolysing NaOH solution with SS (stainless steel) electrodes (304 alloy works fine in that setup, 316 is not needed). They helped me keeping H2
and O2 separated.
|
|
|
experimenter_
Harmless

Posts: 38
Registered: 18-2-2016
Member Is Offline
Mood: No Mood
|
|
The points you made are right on spot Bezaleel. Your last point about the readily available membranes opens up many new possibilities. Obviously you
have some good hands on experience on electrolysis, thanks.
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Quote: Originally posted by Bezaleel  |
* Use the membrane of a lead-acid battery. You'll need the old, wet type of battery. I used the membranes from these batteries with great success
electrolysing NaOH solution with SS (stainless steel) electrodes (304 alloy works fine in that setup, 316 is not needed). They helped me keeping H2
and O2 separated.
|
You mean like these batteries?
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
macckone
Dispenser of practical lab wisdom
    
Posts: 2168
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
Those are gel type. Not all wet types have separators. Most likely are deep cycle wet cells.
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
Quote: Originally posted by elementcollector1  | Quote: Originally posted by Bezaleel  |
* Use the membrane of a lead-acid battery. You'll need the old, wet type of battery. I used the membranes from these batteries with great success
electrolysing NaOH solution with SS (stainless steel) electrodes (304 alloy works fine in that setup, 316 is not needed). They helped me keeping H2
and O2 separated.
|
You mean like these batteries? |
No, I think these are the newer type of dry cells. As far as I know, wet batteries always have compartments (cells) that can be refilled with water.
They have screw caps on top of them for each cell. In my case the screw caps needed to be opened with a philips screw driver, see the picture.

Edit:
Thanks, Macckone. I didn't know this, so I have been lucky opening one that did have the separators.
[Edited on 17-4-2016 by Bezaleel]
|
|
|
macckone
Dispenser of practical lab wisdom
    
Posts: 2168
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
Yes that is a deep cycle from the picture.
One note on the three compartment system is that three compartment cells are used to
Recycle sodium sulfate into sodium hydroxide and sulfuric acid. Commercially they use
Membrane cells that are ion specific but there is no reason a diaphram cell could not do
The same thing to produce hydroxide and bisulfate. This could lead to otc sulfuric acid.
Epsom salt and baking soda yield sodium sulfate. Both are readily available in all but the
Worst war zones.
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1640
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
With out reading the whole thread I used a simpler combustion process, I built this system at 15 when I was far less interested int he science then I
am now days.
I used a mercury cell: A tank with a partial divider, bottom filled with mercury till sealed. The tank was built to allow max surface area with
minimal depth so it only took a few grams of mercury to accomplish the seal. Bottom of tank had a 316L SS nut and bolt and a second nut to attach the
wire.
the brine tank, Same bolt configuration, but the positive electrode was finely coiled SS welding wire that was 1.5 inches from the bottom of the tank.
Tank was made of aquarium glass (When I was fifteen I had a weird obsession with making 2/300g terrariums so had ample glass working gear)
Top plate was machined to allow fluid in and the other side gasses out then it was epoxied on.
these tubes where led to water flash back arresters I had made out of film containers.
These then led to a large glass jar where the ends met at a spark plug, the third hole was the exhaust that led to ice water. The spark plug was
driven by a high voltage supply and it was started first.
1 - Starting sequence: HV supply to the spark plug
2 - Check gas valve leading reactor to ensure they where off
3 - Start cell, and allow to build some pressure.
4 - Crack the chlorine line open and purge line
5 - Slowly open H2 line and then throttle up the chlorine till both where fully open and a self supporting flame was present
6 - Shut down the HV line to the spark plug.
That's all I really remember other then it worked well, I think now days I'd use a chromatography column with a couple 30guage hypodermic needles and
BBQ sparker to kick off the reaction
Take the column and at the reservoir side put in a rubber stopper and push the needles in so the tips just meet.
run the discharge side to the ice water
precharge the column with Cl2 then once flooded start admitting the H2 and ignight to form the HCL. Should make a much more compact and efficient
generator.
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1640
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by hissingnoise  | | Quote: | | Someone else suggested it is easier to make H2SO4 from the SO2 and then distill HCl out of NaCl. Hm, maybe. |
Jeez, am I missing something here . . . ?
Just to be clear, you can simply toss some salt into a flask, add enough conc. H2SO4 to cover it (reaction is immediate and
vigorous) and dissolve the evolved HCl gas in water using an inverted funnel, et voilà!
When the initial reaction slackens, heating the sol. will drive it to completion!
And using distilled water gives pure acid as only HCl comes over . . .
|
Why waste Sulfuric when you can do it super cheap with electricity and a cell? Only real consumables become water and salt!
|
|
|
| Pages:
1
2 |