sclarenonz
Hazard to Self
 
Posts: 74
Registered: 13-12-2015
Location: BRASIL,oiapoque ,amapa
Member Is Offline
Mood: only the mission forget past
|
|
magnesium nitrite or calcium nitrite?
this experiment is proved by min yourself, can do at home and make your observation , first potassium reacts spontaneously form at room temperature
with oxygen , the right amount is trial and error , you will put an amount of magnesium or potassium calcium that over time you will realize that a
part of the potassium was not white , it means that u have to always put a quantity of potassium more, how to get out the oxygen , so will be the 99%
nitrogen along with other minor gases , now is already very easy to leave the stable nitrogen , formerly I was very demented was giving shock in the
air with flyback , without separating the gas , but then I had a better understanding saw that in addition to separate the gases , also need little
energy to leave stable nitrogen , let's look at the nitrogen of the spectrum in its absorption , cause the nitrogen so will absorb electrons to form
their stable octet, observe the following:
http://www.deboni.he.com.br/dic/quim1_007.htm
note that green is what the nitrogen absorbs more nitrogen is from 5 to family , now let's look at an element of the periodic table that emits green:
of all the one who is very selective in its issuance is the magnesium you need emit electrons to form their stable octet =
http://www.deboni.he.com.br/dic/quim1_012.htm
note that the magnesium is sending the same range of frequency of nitrogen, and besides he sent it absorbs in the same range tb, it is not incriviel,
and the similarities do not stop, the magnesium is in the same opposite family of nitrogen, it's like were his pair on the opposite side, the
magnesium reacts in a way slowly with oxygen, but in the right range spontaneously reacts with nitrogen. means that do not need a lot of energy so
that the nitrogen reacts with the magnesium, it means that if we put an electric charge on magnesium it will emit electrons at the same frequency of
nitrogen attracting up to you, now we will solve the issue, after all the oxygen react with potassium or alkali metal, we put the magnesium react with
nitrogen, but the difficulty is only found easier the magnesium and potassium in forms of oxide and carbonates, as we separate the two oxygen metals.
first let's see what wikipedia says the potassium =
The significance of the discovery is the confirmation of Antoine Lavoisier hypothesis that soda and potash reacted with acid in the same manner that
the oxides of lead and silver, they were formed of a metal combined with oxygen, finally confirmed the isolation potassium, and a week after the
sodium by electrolysis of soda. Moreover, obtaining potassium enabled the discovery of other elements since, due to its high reactivity, is capable of
decomposing oxides oxygen removing them thus were isolated silicon, boron and aluminum.
Now we need to give attention this:
https://en.wikipedia.org/wiki/Magnesium_nitride
`In fact, when magnesium is burned in air, magnesium nitride is formed along with magnesium oxide in their maioria`` picture without oxygen then
another thing
Magnesium reacts with carbon dioxide to form magnesium oxide and carbon:
Mg 2 + CO
2 → MgO + C 2 (s)
Thus, carbon dioxide fire extinguishers are ineffective for magnesium fire extinguishing.
now another question:
https://en.wikipedia.org/wiki/Potassium_carbonate
Potassium carbonate (K2CO3) is a white salt, water-soluble (insoluble in ethanol) [2], which forms a strongly alkaline solution. It can be made as the
product of the absorber reaction of potassium hydroxide with carbon dioxide. It is deliquescent, often appearing a damp or wet solid. Potassium
carbonate is used in the production of soap and glass.
then I leave the question the nature choose the potassium or magnesium to break the co2
she would choose the potassium or magnesium to break the nitrogen
the answer comes here
https://en.wikipedia.org/wiki/Potassium_nitrite
see that nature can not form nitride potassium without oxygen and nitrogen can not react with the potassium, it is not interesting !, two elements in
electronegativity matter the academic world says that reacts in the real world the potassium reacts not with the nitrogen, the nitrogen has to react
first with oxygen and then the oxygen react with potassium, we know that the reaction of nitrogen with oxygen is very exothermic, nature would never
choose this path, this only happens in the spokes, or places that it rains a lot, we have potassium nitrate in the soil.
today we show in practice that the academic world is really a complete farce.
Now we started to do the little experience
look for a non colorless white mineral 70% of cases will have an alkaline earth, remember that if colorless red has chlorine is alkaline, is white
goal with alkaline and alkaline transparent white has with oxygen =
https://es.wikipedia.org/wiki/Magnesita
look for the best alternative
https://es.wikipedia.org/wiki/%C3%93xido_de_magnesio
now we have the magnesium oxide,
now the hydroxide of potassium, but if in your region is easier to find the hydroxide of sodium can use is the same family, if not find the magnesium
oxide may be the oxide calcio, now look as it does to isolate the calcium or magnesium, this example with magnesium and potassium is because of the
comparison with the photosynthesis of plants.
hydroxide smaller box sodio
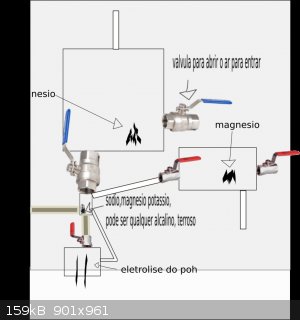
first opens the valve for the air enters, closes all valves, the potassium of the smaller box will react with any oxygen to turn potassium oxide, now
closes all valves are going to be the magnesium in the larger box now from it a high-voltage shock magnesium, magnesium anger react with nitrogen to
generate nitride of magnesium which is a yellow solid, now only will remain as carbon dioxide, water enters the lower box and potassium oxide turns
hydroxide of potassium, now the carbon dioxide is thrown to the smaller box where the potassium oxide, carbon dioxide is soluble in water closes the
valve of the smaller box, fully enclosed simply shakes with great intensity, the oxide of potassium anger regir with carbon dioxide to form water and
potassium carbonate. \
the potassium carbonate reacts with water to form sodium bicarbonate. playing hydrogen gas know we methanol. with magnesium catalyst and not the usual
iron to attract the best oxygen yet.
the portion of potassium bicarbonate with magnesium I have to test. but that part does not matter much not have much interest in taking carbon dioxide
but summarizing why the interest, imagine a nitrogen reactor that could fit in the palm, which does not warm up, imagine nitride magnesium, with
stable nitrogen, nitrogen to molecule of the death of one combustion 100 times in our body making the blood move 100 times faster (drug) and an
explosive component, imagine it in the palm of the hand, the iron man of the dream is not a lie is true.
|
|
|
|