tomtailford
Harmless

Posts: 1
Registered: 19-11-2006
Member Is Offline
Mood: No Mood
|
|
Decarboxylation of amino acids
Quite simply I would like to remove a carboxylic acid group (COOH) from a amino acid (X) - there are different processes but due to limited
availability of certain chemicals I am hoping I can use PCl5 with LiAlH4 or NaBH4.
So the equation is as follows:
X-COOH ---PCl5 / Reducing Agent---> X-H
My questions are as follows:
1. What sort of mixtures would you have to use, ie. does the generic amino acid have to be dissolved in anything? Same goes for the PCl5 / Reducing
agent? Does anyone have a simple procedure which I could use and follow?
2. I take it the amounts of X-H produced can be worked out from the X-COOH used in the first place.
3. If there is any optical activity in the amino acid you start with, will it remain the same isomer?
[Edited on 19-11-2006 by tomtailford]
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
...................after you've searched here for an answer to your question............here is one search result........try it it works.......
https://sciencemadness.org/talk/viewthread.php?tid=3270
you might try looking here,
Check the Hive Archives , rhodium posted a one step method to reduce the COOH to the CH3 in one step..................
http://www.telegenetic.net/hiveboard/
or search......the rhodium pdf's are here,
http://www.erowid.org/archive/rhodium/pdf/?S=D
and the rhodium archives can be found here.....
http://www.erowid.org/archive/rhodium/
..................bottom line you need to do some research, the answers have been archives ...........................solo
[Edited on 20-11-2006 by solo]
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
Drunkguy
Hazard to Others
  
Posts: 172
Registered: 23-12-2005
Member Is Offline
Mood: somewhat pissed.
|
|
Why dont you make sum Deprenyl instead?
Loooooool 
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Where does Deprenyl fall in the scheme of CNS activity ........as it may not be worth the trouble as such...............solo

[Edited on 21-11-2006 by solo]
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
Elawr
Hazard to Others
  
Posts: 174
Registered: 4-6-2006
Location: Alabama
Member Is Offline
Mood: vitriolic
|
|
Deprenyl, AKA selegiline, is a MAO inhibitor used for decades in treatment of Parkinsonism and as a nootropic. Recently, a transdermal form has been
made available for treatment of depressive disorders. Because the transdermal route bypasses the portal circulation (the gut) many of the harmful
food/drug interactions associated with MAOI therapy are avoided. It is said to be highly efficacious for mood disorders even those refractory to
other antidepressants. However, a months supply of this product Emsam costs hundreds of dollars.
So yes, absolutely - an easy synthesis for clean selegiline would be of extreme interest to me both as a clinician and as a sufferer of dysthymic
disorder. This is not a controlled substance, after all.
[Edited on 20-11-2006 by Elawr]
1
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Ok, lets keep this just to the chemistry of such substances, the potency of such is not for here. There are other places on the web for discussion of
such effects.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by tomtailford
Quite simply I would like to remove a carboxylic acid group (COOH) from a amino acid (X) - there are different processes but due to limited
availability of certain chemicals I am hoping I can use PCl5 with LiAlH4 or NaBH4.
So the equation is as follows:
X-COOH ---PCl5 / Reducing Agent---> X-H
|
What the hell are you talking about? What does PCl5 and LiAlH4 have to do with the decarboxylation of amino acids and what kind of nonsense is the
above equation? Decarboxylation of amino acids is not an intermolecular redox reaction and thus it does not require oxidants or reducents. Utmost it
is an intramolecular disproportionation in regard to the oxidation/reduction issue.
Anyway, putting PCl5 and LiAlH4 together is a sure route to a small volcano that would put your lab in fire and you in the hospital (in case you
survive – depends on the reaction scale). So make sure there will be no others in the building when you do that!
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
I think he means to remove the COOH to a Cl with PCl5 which will not happen, although both LIAlH4 and NaBH4 will reduce tht COOH to an alcohol.
Nothing of the kind will happen by adding PCl5 along , maybe after the alcohol is made then it can be halogenated with the aford said acid or thionyl
chloride .............as I see it,.......solo
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
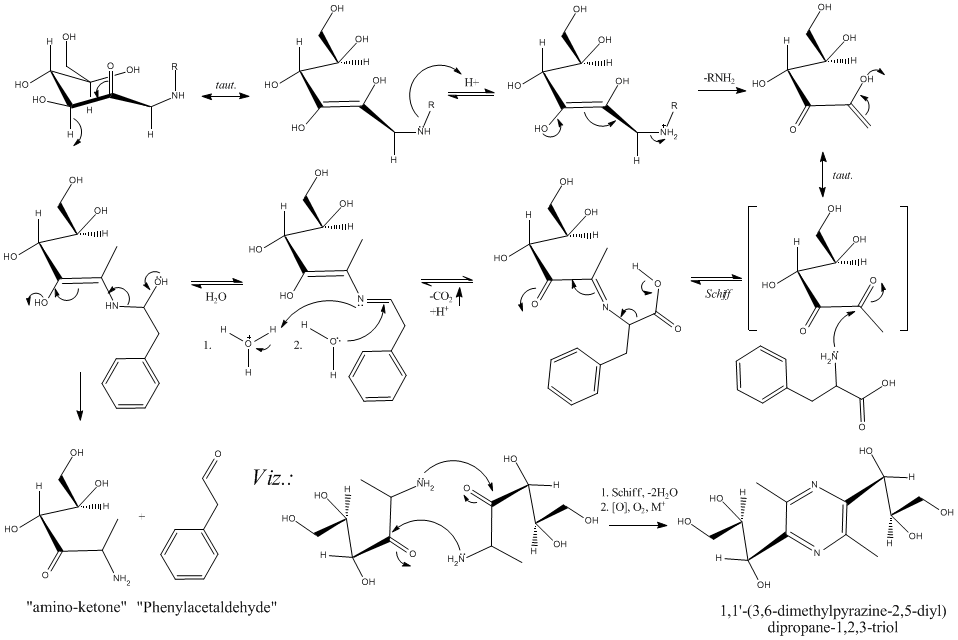
decarboxylation of L-phenylalanine (Phe) via...Glucose
Hello All,
I alluded to this on the "Benzaldehyde" thread as phenylacetaldehyde is also interesting.
If you want to easily decarboxylate an amino acid, you must first "schiff up" the amino group. In doing this, resonance is set up via the imine
enabling the decarboxylation to proceed under much more mild conditions. This happens (and I have done this several times) in mixtures of glucose (50%
w/w, say) with added L-Phe held at, say, 80°C for up to 10 hours (action usually follows the initial browning of the mixture in about 1hr). The
phenylacetaldehyde that is formed is easily detected by smell (which is quite nice) or via the DNPH derivative. Increasing the temperature whilst
maintaining a pH between 7-8 will drive this, but lead to losses in product (condensation, aldol).
This does *not* yield phenethylamine. It does give the aldehyde analogue (see "Strecker degradation", and the "Maillard reaction", J. Agric. Food.
Chem. (ACS) has some good references, also the original paper by Hodge (1953) in JACS) and the corresponding amino ketone (which can lead to novel
pyrazines).
This is, at least, within the confines of the title of this post. This mechanism, if carefully considered, can likely be manipulated to *not*
deaminate as well.
Included is the mechanism for this starting with D-glucose and L-Phe (the pyrazine addition is lagniappe, and multiple steps).
Easy, yes, multiple products? (at this time yes), but *cheap*...yes.
Hope this is interesting. Are there Any thoughts on a carbohyrate Chemistry thread under either Organic, or more likely, Biochemistry?
Cheers,
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
jusdel30
Harmless

Posts: 6
Registered: 8-7-2017
Member Is Offline
Mood: No Mood
|
|
Is the precipitate primarily phenylacetaldehyde that appears after an hour or so into the reflux?
[Edited on 8-7-2017 by jusdel30]
|
|
|
jusdel30
Harmless

Posts: 6
Registered: 8-7-2017
Member Is Offline
Mood: No Mood
|
|
obviously not as the aldehydes a liquid no? the crust on top is the product of the maillard reaction i assume? just trying to work out a way to
separate is all. end products should all be around 7-8 ph wise?
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
since when did glucose have a secondary amine group ?
| Quote: | | This mechanism, if carefully considered, can likely be manipulated to *not* deaminate as well. |
IMO,you could add a very weak reducing agent,like STAB, after the decarboxylation step.It would reduce the imine rather than the enamine, since the
former is less stable,so more able to react with STAB and also due to steric hindrance.Then the enamine could be hydrolysed to get phenethylamine.
[Edited on 9-7-2017 by CuReUS]
|
|
|
sparkgap
International Hazard
    
Posts: 1234
Registered: 16-1-2005
Location: not where you think
Member Is Offline
Mood: chaotropic
|
|
I was a bit confused by the diagram myself, but it seems the idea is to use phenylalanine to form an imine with glucose. Since Phe's isoelectric point
is 5.5, the amine portion ought to condense with glucose after some heating. Still looks messy tho.
sparky (~_~)
"What's UTFSE? I keep hearing about it, but I can't be arsed to search for the answer..."
|
|
|