vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
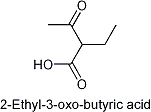
Ethylacetoacetate...
.....or 2-Ethyl-3-oxo-butyric acid.
1) To which class of organic compound does this compound belong? Keto-acids?
2) Does anybody have an idea about the synthesis?
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
"Ethylacetoacetate" generally is used to refer to the ethyl ester of acetoacetic acid.  I believe the compound you've targeted for synthesis is considered a keto acid. I believe the compound you've targeted for synthesis is considered a keto acid.
What starting materials are you allowing yourself, and how involved of a synthesis are you looking for? You could prepare that compound via a Claisen
condensation of a mix of ethyl acetate and ethyl n-butyrate. Ethyl n-butyrate could be prepared by Favorskii rearragement (in this case, treating
1-chlorobutan-2-one with sodium ethylate - you might have trouble preparing that from MEK, though, due to chlorination of MEK yielding lots of
3-chlorobutan-2-one - at least it's OTC, though) or from esterification of n-butyric acid (but you'd have to buy that from a chemical supply
house).
I weep at the sight of flaming acetic anhydride.
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
Ester? That's not an ester. There is no R-O-CO-R bond.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
Bugger, the structure I posted is incorrect. I obtained it by using the convert name to structure command of Chemsketch. No idea why it fucked up.
It's intended use is the Belousov-Zhabotinsky reaction, so the product should contain as less chloride as possible.
I was investigating if it was plausible to make it yourself from OTC compounds, but it seems not. If I have to buy the precursors I'd better buy
the stuff itself because it's not expensive at all.
Ofcourse it would be fun to synthesize it myself..
[Edited on 15-6-2003 by vulture]
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
You could make the required strong base by reaction of calcium oxide with anhydrous ethanol, making ethyl acetoacetate synthesis OTC (distill ethyl
acetate out of nail polish remover).
I weep at the sight of flaming acetic anhydride.
|
|
|
Mendeleev
Hazard to Others
  
Posts: 237
Registered: 25-12-2003
Location: USA
Member Is Offline
Mood: stoned
|
|
Most syntheses such as this which require anhydrous ethyl acetate call for drying it by refluxing with P2O5, however on Rhodium's ethyl
acetoacetate synthesis, one purification method states that merely drying with granular CaCl2 overnight is sufficient. Is CaCl2 really enough? In
that case why waste phosphorus pentoxide?
Trogdor was a man. A dragon man. Or maybe just a dragon. . .
|
|
|
alchemie
Harmless

Posts: 36
Registered: 4-6-2003
Member Is Offline
Mood: variational
|
|
Do you need to use the oxobutyric acid specifically? Every procedure I see on the net uses malonic or citric acid.
This site has a lot of pdf articles about cyclic reactions and lots of other interesting stuff about the general theme of chaos and complex systems.
Some of the articles concern the Belousov-Zhabotinsky directly.
http://chaos.ph.utexas.edu/entire.html#ChemicalInstabilities
[Edited on 13-12-2004 by alchemie]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
ethyl acetoacetate
I made some of this ester recently using the Claisen condensation of ethyl acetate using sodium ethoxide. It is interesting in that it requires
elemental sodium as a reactant to make the sodium ethoxide in situ from generated ethanol. This depends on the charged ethyl acetate having
a few percent of ethanol to get things started.
I started with 117mL of ethyl acetate and 9.25g of Na cut into slivers. This is refluxed for about 1.5 hours. A carmel colored solution results
(color due to some tar generation apparently).
During the workup my procdure called for a wash with saturated aqueous NaCl. I misread this and used a saturated aqueous solution of CaCl2. What
resulted was a 500mL separatory funnel nearly full of white gel!  After
feeling very discouraged for awhile I decided, what's to lose, I might as well try a salvage operation. So added enough water to get the gel to
dissolve, separated off the water, then did a NaCl wash. After
feeling very discouraged for awhile I decided, what's to lose, I might as well try a salvage operation. So added enough water to get the gel to
dissolve, separated off the water, then did a NaCl wash.
Final purification was by simple distillation into 3 cuts, with redistillation of the middle cut. The procedure said yields could be improved by
vacuum distillation. My hydroaspirator seemed to lock onto about 63 mmHg absolute. So that is what I used and the final portion came over steadily
at 95C. Normal bp for the ester is 181C.
My yield was 13mL, or 16.7%. As the procedure said only 6.5 mL would be expected by distilling at atmospheric pressure I was pleased.
This ester supposedly exists at 7% in its enolic form. This allows a qualitative test with ammoniacal CuSO4 that forms an aquamarine salt precipitate
with the enol. This precipitate dissolves in chlorofrom. These tests on my ester were positive. Vogel also describes an interesting test with FeCl3
but I don't have any so couldn't do this.
This ester can supposedly be easily alkylated, hydrolyzed, and then decarboxylated to form various methyl ketones. I may try this with butyl methyl
ketone being my most likely synthetic target.
[Edited on 15-2-2007 by Magpie]
[Edited on 15-2-2007 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
[Edited on 16-2-2007 by chemrox]
|
|
|
benzylchloride1
Hazard to Others
  
Posts: 299
Registered: 16-3-2007
Member Is Offline
Mood: Pushing the envelope of synthetic chemistry in one's basement
|
|
Can methyl acetoacetate be produced by using magnesium methoxide which can be easily produced from dry methanol and magnesium powder? This would be a
useful synthesis if it works because it would eliminate the need for expensive sodium metal. I chose the methyl ester because anhydrous methanol is
readily available, along with ethyl acetate. You would end up with mixed esters, but this would not be a problem for synthesis of other compounds
where the ester functional group is lost in the reaction.
Amateur NMR spectroscopist
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Magnesium methoxide is a substantially weaker base than sodium methoxide, particularly in nonpolar solvents, due to the higher acidity of the
Mg<sup>2+</sup> cation when compared to Na<sup>+</sup>. But in principle it should work as long provided the reaction times
are longer or conditions harsher. Try with a reflux of ethyl acetate in xylene with dried Mg(OMe)2 (or with the more soluble Mg(OEt)2), or some
similar harsh conditions. Methanol (or ethanol) should be removed in the azeotrope and you should be left with the suspension of magnesium (m)ethyl
acetoacetate salt.
But why not using NaOEt prepared from NaOH and EtOH? There are several methods for preparing NaOEt from NaOH and none is particularly difficult. See
the threads on topic.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|