gregxy
Hazard to Others
  
Posts: 421
Registered: 26-5-2006
Member Is Offline
Mood: No Mood
|
|
Preparing ethyl glutathione esther
I am interested in preparing di ethyl glutathione esther
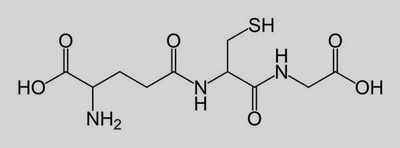
Glutathion (see below) is a small peptide with carboxyl groups at each end. Any suggestions on how to prepare
a di-ethyl esther without destroying the glutathion would be appreciated.
Thanks
http://en.wikipedia.org/wiki/Glutathione
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Glutathione is, to be exact, a tripeptide, made up of three amino acids. The conplicating factor is the presence of a sulfhydrl (thiol) group which
you will have to protect before esterifying the carboxyls, and afterwards you will have to deprotect them. The choice of PG depends on what your
esterification step will be, and the choice of deprotecting reagent will deend on which will leave your ethoxy groups alone. Fun, isn't it?
Glutathione is gamme glutamylcysteinylglycine or Glu-Cys-Gly. The aggravating -SH is on the Cys of course.
Esther by the way is a woman's name, and a book of the bible (old testament) but what you mean is ESTER which is a different matter entirely. 
Anyway I suggest you get two books by Bodanzsky, Principles of Peptide Synthesis" and Practice of Peptide Synthesis" both 2nd edition, published by
Springer Verlag.
Sulhydryl groups by the way are, in peptide chemistry, a pain in the ass.
[Edited on 17-2-2007 by Sauron]
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
If that -SH is unreactive to TCT (cyanuric chloride) you can skip the protection and prepare the acid chloride that wat, with TEA in acetone, and
esterify with EtOH in situ.
One mol TCT to one mol of glutathione, as TCT has two very active Cl (plus one not so active.)
M.Falloum in his 1999 Tet.Lett. article on cyanuric chloride for preparation of acid chlorides demonstrated the applicability to amino acids, and
oligopeptides by preparing esters and amides from them in situ. I do not remember if any of these inclided cysteine or methionine residues.
This is a facile room temperature process involving an easily handled solid reagent. A lot easier than protection/esterification/deprotection.
[Edited on 17-2-2007 by Sauron]
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
I found a reference that indicates that TCT can be used to activate carboxylic acids to facilitate preparation of esters of alkyl and aryl thiols, All
they are saying here is that TCT made the acids into acid chlorides in presence of a tertiary base (they used N-methylmorpholine, but no matter, it
could have been TEA or pyridine) and these reacted in situ with thiols. Activation, schmactivation.
Therefore, it is possible that one of the "activated" carboxylic terminals of glutathione "might" react with the -SH of a second glutathione molecule
and produce a hexapeptide before your ethanol can esterify them.
So most likely you could lose some glutathione to this side rxn.
Unless you use a PG on that sulhydryl function, before you add TCT, and ethanol, and TEA; then after working up your protected diethyl glutathione,
knock off the PG. That is what PGs are for, after all.
Selecting the "best" PG reagent, now, that is where the peptide chemistry begins.
|
|
|
Furch
Hazard to Others
  
Posts: 130
Registered: 8-10-2006
Member Is Offline
Mood: No Mood
|
|
I couldn't find the article you referred to. Anyway, I think it's safe to say that if you convert the acid groups to acid chlorides, they will be
utterly prone to react with the strongly nucleophilic thiol group... I think you have to find another way. Protection of the thiol
group sounds like a good idea!
\"Those who say do not know, those who know do not say.\" -Lao Tzu
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
If you go look in the Wanted References thread (assuming you have the PW to References) you will see it is one of the most recent postings. The
article was by a couple of Indian chemists in Journal of Sulfur Chemistry.
If you don't have the PW, I'll post the article here but I'd rather not have to put it up twice on the forum, which has finite hosting capacity. So
after those who are interested download it I'll take it down in a day or two.
It is an extension of the usefulness of TCT after all.
Acids to acid chlorides to esters, amides, anhydrides and thioesters
Alcohols to alkyl chlorides and alkyl bromides.
Useful stuff.
|
|
|
Furch
Hazard to Others
  
Posts: 130
Registered: 8-10-2006
Member Is Offline
Mood: No Mood
|
|
I ment Falloums Tet. Lett. article from 1999. But nevermind. Thanks anyway though... And sorry for not reading throught your posts more thoroughly
before replying *ashamed*
Good luck anyway!
\"Those who say do not know, those who know do not say.\" -Lao Tzu
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Not to worry, I am having trouble finding that article myself. I think I spelled his name wrong, I think it is Falomi or Faloumi. He and colleagues
had a number of articles in Tet.Lett on CC/TCT and I have this one on hand somewhere here in hard copy
When I find it I will scan and post it. What I have was off of Rhodium way back, and is not to be found in the Erowid partial archive.
|
|
|