stoichiometric_steve
National Hazard
   
Posts: 819
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
now for some retrosynthesis: 3-[N-diethyl-1-(n-propanamide)] on a primary nitrogen.
QSAR studies say that an amphetamine like DOI coupled with a 3-[N-diethyl-1-(n-propanamide)] on the nitrogen makes a 5HT2a receptor agonist equipotent
to LSD.
how could the synthesis be accomplished, starting from the amphetamine?
|
|
|
Furch
Hazard to Others
  
Posts: 130
Registered: 8-10-2006
Member Is Offline
Mood: No Mood
|
|
I would love to help you, but I don't understand what the molecule looks like. 3-[N-diethyl-1-(n-propanamide)] makes no sense to me... Can you perhaps
draw the molecule, or give me the SMILES code for it?
\"Those who say do not know, those who know do not say.\" -Lao Tzu
|
|
|
stoichiometric_steve
National Hazard
   
Posts: 819
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
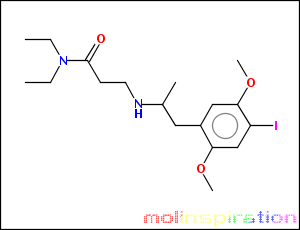
the SMILES for the complete molecule would be
and the correct IUPAC name N,N-diethyl-3-(1-(4-iodo-2,5-dimethoxyphenyl)propan-2-ylamino)propanamide
CCN(CC)C(=O)CCNC(C)Cc1cc(OC)c(I)cc1OC
see attachment for picture.
[Edited on 13-3-2007 by stoichiometric_steve]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by stoichiometric_steve
QSAR studies say that an amphetamine like DOI coupled with a 3-[N-diethyl-1-(n-propanamide)] on the nitrogen makes a 5HT2a receptor agonist equipotent
to LSD. |
Do you have in mind a quote from Nichols, in some old paper I just can not find at the moment, who once hypothesized this compounds might be more
potent 5-HT2A agonists than the N-unsubstituted parent 2,5-dimethoxy-4-X-phenylethylamines? His hypothesis was not based on QSAR, but plain good old
SAR (QSAR is completely useless for such predictions). If you have some other reference, please be a gentleman and provide it.
Anyway, now, years later when the super potent psychedelic N-(2-methoxybenzyl) phenylethylamine and tryptamine derivatives were discovered, part of
his hypothesis could be considered proven (2-methoxybenzyl isostere efficiently acts as the talked about alkylamide group at the receptor). But to my
knowledge the N-(CH2CH2CONEt2) substitution was never tried on an 5-HT2A agonist. Thus it is formally still just a hypothesis.
It should be possible to alkylate N-benzyl protected phenylethylamines with methyl acrylate, followed by hydrolysis, coupling with diethylamide and
debenzylation with H2/Pd-C. Perhaps it would be even possible to alkylate directly with N,N-diethyl acrylamide which is however considerably less
electrophilic than methyl acrylate. This would allow skipping the hydrolysis and coupling steps.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
stoichiometric_steve
National Hazard
   
Posts: 819
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
the ref is:
Schulze-Alexandru, Kovar, Vedani:
Quant. Struct.-Act. Relat., 18 (1999) pg. 548-560
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
According to the literature you are lucky in that:
a.) acrylamides are just electrophilic enough for the alkylation of amines;
b.) there are a couple of examples where primary amines were selectively monoalkylated with acrylamides.
Therefore, there is no need for amine benzylation to preform the secondary amine and you can use straight N,N-diethyl acrylamide for the
alkylation. This means the synthesis is a one step reaction from the appropriate phenylethylamine, though you need acrylic acid chloride and
diethylamine to prepare the required electrophile.
That paper (attached) looks a bit dull like most QSAR papers and it does not seam to give the credit to Nichols for the original suggestion of the
-CH2CH2CONEt2 substitution at the amine. However, I have to admit they were quite advanced with their interaction modeling considering that it is from
the year 1999 when Heim's 2-methoxybenzyl amine substitution was yet unknown. Yet, given that such an amide might be metabolically somewhat less
stabile and certainly much more hydrophilic, I would expect any such compound like you draw would be less potent than the N-(2-methoxybenzyl)
corresponding counterpart (the N-(2-methoxybenzyl)-2C-B is expected to be psychedelic at about 200 micrograms without considering the
metabolism, but more likely at about 500 micrograms given that benzylamines tend to be slowly oxidatively cleaved in vivo). Affinity, and
moreover predicted affinity is not all that is required for high potency. Besides metabolism, BBB penetrability and other pharmacodynamics you
need to consider also the possibility that the compound might be an antagonist. The N-(2-methoxybenzyl) compounds were luckily found to be
agonists, but their intrinsic activity is reduced in comparison to the parent compounds (for example, 2C-B has the intrinsic activity of 58% relative
to serotonin, N-(2-methoxybenzyl)-2C-B has it 38% while for N-(2-methoxybenzyl)-DOB it drops to 20% which nearly fits for a partial
agonism). The -CH2CH2CONEt2 group has much more "degrees of freedom" (less restricted in its conformational transformations) than the 2-methoxybenzyl
group, so I would expect an even more radical drop in the intrinsic activity (probably to the degree of a low efficiency partial agonism).
Yet a very interesting new type of compound to try and see…
Attachment: QSAR receptor surrogates for the 5HT2A receptor.pdf (320kB)
This file has been downloaded 713 times
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
stoichiometric_steve
National Hazard
   
Posts: 819
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
hmmm, interesting. i never heard or read about the 2-methoxybenzylated stuff. have you got any good reads in that area? i'd highly appreciate that.
thanks in advance 
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
http://www.diss.fu-berlin.de/2004/81/index.html
http://molpharm.aspetjournals.org/cgi/content/abstract/70/6/...
Naunyn-Schmiedeberg's Arch. Pharmacol. 2002, 365 (Suppl. 1), R29 & 2003, 367 (Suppl. 1), R31 (attached)
Also related: FR2181559
|
|
|