| Pages:
1
2
3 |
DIBALL
Harmless

Posts: 25
Registered: 4-1-2017
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Boffis  | @ Diball; when you heat glycine with glycols you get cyclitisation to to piperazine-2,5-dione. Alanine yields the 3,6-dimethyl analog. Once formed
these compounds cannot form a Schiff base type condnsation product so I suspect that once formed these compompounds resist the effects of a ketone.
The formation of these piperazines should occur with any alpha amino acid but in practice only the simplest of the amino acid undergo this type of
reaction as far as I am aware.
I think that shive_inorganic is onto something with his sodalime degradation. Has anyone tried heating calcium glycinate and calcium oxide to
decomposition? The idea of the extra calcium oxide is to remove an extra CO2 and reduce the formation of C3 compounds (as in the case of acetone from
calcium acetate).
Diball, in your last post how did you determine that the gas released was ammonia? Methylamine smells much like ammonia. |
Alright, so my first reaction failed because I made piperazine-2,5-dione. That makes sense!
I remember the smell of methylamine from my last formaldehyde/ammonium chloride synthesis as a substance, that smells like fishy cat piss. This one
was smelling like ammonia. But now that I think about it, it would make more sense for the glycine to release MeNH2 instead of ammonia!
Is there a way to test, if it is actually MeNH2 and not ammonia?
The calcium route sounds interesting. Magnesium should work as well?
[Edited on 16-3-2018 by DIBALL]
|
|
|
Chemi Pharma
Hazard to Others
  
Posts: 349
Registered: 5-5-2016
Location: Latin America
Member Is Offline
Mood: Quarantined
|
|
I suspect this kind of decarboxylation of glycine with sodalime will evolute to the decomposition of any methylamine generated in situ to HCN, methane
and ammonia, like said in the paper I attached.
So, I think these concurrent reactions take place, based on what I read in "The Hive" on the NET, and what the paper say:
NH2CH2COOH + 2 NaOH --> Na2CO3 + H2O + CH3NH2
CH3NH2 ---> HCN + 2 H2
HCN + NaOH --> NaCN + H2O
CH3NH2 + H2 --> CH4 + NH3
Overall reaction:
3 NH2CH2COOH + 7 NaOH --> 3 Na2CO3 + NaCN + 4 H2O + 2 CH4 + 2 NH3
Maybe because this fact @Diball sniffed only ammonia smell instead methylamine, while heating glycine and sodalime in the test tube.
Attachment: thermal decomposition of methylamine.doc (154kB)
This file has been downloaded 393 times
[Edited on 16-3-2018 by Chemi Pharma]
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
No, propylene glycol is not your problem. It works just fine as a solvent for decarboxylation using an enone catalyst. Your issue seems to be
misreading a single vowel in the writeup; you need cyclohexenone, which is not very easy at all to find. What you probably have is
the much more common cyclohexanone. But all is not lost. Spearmint oil contains carvone, which is a suitable enone for this
decarboxylation. However, this reaction may not work with glycine, as I've never tried it. It will work with the higher MW ones though.
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
Pumukli
National Hazard
   
Posts: 686
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
"@Diball sniffed only ammonia smell instead methylamine"
Can anyone tell the difference by smell between NH3 and methylamine?
When I smelled dimethylamine for my nose it was 100% as if it was ammonia. I suspect that the difference between methylamine and ammonia smell is even
smaller if any.
Also, my gut feeling is that the process:
CH3NH2 ----> HCN + 2 H2
is not particularly favoured thermodynamically. Much less under the circumstances of decarboxylation. Maybe at higher temperatures it can be
significant.
Edit: yepp, first I wrote my posting, then I downloaded your reference. It definitely talks about HIGH temperatures!
When will you reach 500-550 C (not to mention 1200) during a decarboxylation, especially in a test tube?
[Edited on 16-3-2018 by Pumukli]
|
|
|
Chemi Pharma
Hazard to Others
  
Posts: 349
Registered: 5-5-2016
Location: Latin America
Member Is Offline
Mood: Quarantined
|
|
I had sniffed ammonia vapours and also methylamine vapours many times during some synthesis. The two smells are completely different I can assevere
you this @Pumukli.
The paper say what happens at 500ºC with methylamine alone, that decomposes in HCN, methane and ammonia. I have my doubts if sodalime could catalyze
this reaction in much lower temperatures, like 250ºC.
Anyway, I challenge anyone here to prove I'm wrong bringing references that Glycine can produces reliable quantities of methylamine by thermal
decarboxylation with alkalis or earth-alkalines oxides.
Glycine is an aminoacid, the amino group undergoes unstable during a high temperature thermal decarboxylation, finishing to evolving like an ammonia
gas from the reaction vessel. This is the reason aminoacids decarboxylation is usually do by schiff base pyrolisys (at much lower temperatures) or
hydrolisys, formed between their reaction with ketones. I never heard about this kind of decarboxylation and never saw it in any organic chemistry
book I've read.
No way aminoacids decarboxylate the same way carboxylic acids does with sodalime or equivalent and I wonder seeing references that proves this theory.
[Edited on 16-3-2018 by Chemi Pharma]
|
|
|
LearnedAmateur
National Hazard
   
Posts: 513
Registered: 30-3-2017
Location: Somewhere in the UK
Member Is Offline
Mood: Free Radical
|
|
I don’t think either can be readily distinguished by smell due to how similar they are, it would be like trying to differentiate formic and acetic
acid in the same way, both smell like vinegar so it’s pretty much impossible. Plus, the human senses aren’t exactly prime scientific tools, what
one person may experience could be different from others; personally I find that ammonia is a ‘fishy cat piss’ smell as described for methylamine,
plus -CH3 is a fairly inert group so it wouldn’t do much to modify the base odour.
That’s what I was thinking, doesn’t decarboxylation occur around or below 200-300C? Can’t find any references to specific temperatures but I’m
sure that it wouldn’t need to be as high as the decomposition temperature of methylamine. Plus, since it’s being removed as a gas, yields would
only be slightly affected - of course there will be a little contamination if you take a Boltzmann distribution into consideration, but distilling off
methylamine would take it to lower temperature regions so decomposition is retarded. Definitely not a case where the gas is forcefully contained in a
vessel which is at the required temperatures for significant conversion.
In chemistry, sometimes the solution is the problem.
It’s been a while, but I’m not dead! Updated 7/1/2020. Shout out to Aga, we got along well.
|
|
|
Chemi Pharma
Hazard to Others
  
Posts: 349
Registered: 5-5-2016
Location: Latin America
Member Is Offline
Mood: Quarantined
|
|
I agree that olfact is not the most reliable sense the human body have. I can't describe that, but the difference between ammonia and methylamine
smell, FOR ME, is quite singular. The same between formic acid and acetic acid, The both, FOR ME, are unmistakable.
But I think it's overkill when you say: "... plus -CH3 is a fairly inert group so it wouldn’t do much to modify the base odour." Don't say this kind
of thing man. Even one more atom linked to a molecule change everything, including the odour. Is like to claim methyl iodide has the same odour of
iodine, cause is just a -CH3 group attached, isn't it?
I will not discuss more, cause it's a waist of time and efforts. I just want references about the theory you claim to work. Until there I will keep my
position.
|
|
|
Pumukli
National Hazard
   
Posts: 686
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
All right :-) Now I know that NH3 and MeNH2 smell the same. And I also know they are quite different in this regard. :-)
So it seems someone should test it (unless one can find a good reference.)
What would be the best (amateur friendly) method differentiating between NH3 and MeNH2? Some sort of precipitation? Making an amide and determining
its melting point?
|
|
|
LearnedAmateur
National Hazard
   
Posts: 513
Registered: 30-3-2017
Location: Somewhere in the UK
Member Is Offline
Mood: Free Radical
|
|
It depends on the class of molecules, iodine (a halogen where I is in the 0 oxidation state) has VERY different properties to methyl iodide (a
haloalkane with I in the -1 oxidation state) - Cl2 and HCl smell nothing alike because they’re barely reminiscent of each other in terms of
properties, even though they both contain chlorine atoms. On the other hand, both formic and acetic acid are homologous carboxylic acids, and
methylamine/ammonia are homologous amines where the NH2 is the main group in question, and is responsible for the smell of ALL amines.
Anyway, I can’t find a reference to the decarboxylation of a-amino acids using sodium hydroxide, but I did come across this equation which uses
barium hydroxide instead. It comes from this webpage: https://chemistry.tutorvista.com/biochemistry/amino-acids.ht...
I still stand by what I say though, going off the reaction mechanism for decarboxylation.
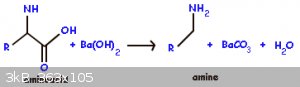 
In chemistry, sometimes the solution is the problem.
It’s been a while, but I’m not dead! Updated 7/1/2020. Shout out to Aga, we got along well.
|
|
|
Chemi Pharma
Hazard to Others
  
Posts: 349
Registered: 5-5-2016
Location: Latin America
Member Is Offline
Mood: Quarantined
|
|
Still waiting for reliable references. 
Come on, this equation is completely theoric. Everyone can write this and Tutorvista are not a worthy site of organic chemistry.
Try to find a research, a study or a reference inside a reliable organic chemistry book. I spent two hours at google searching for that and found
nothing. If you get you deserve my congratulations man.
|
|
|
LearnedAmateur
National Hazard
   
Posts: 513
Registered: 30-3-2017
Location: Somewhere in the UK
Member Is Offline
Mood: Free Radical
|
|
Believe me, if I could find a reference then I would’ve jumped on it immediately, it’s just annoying because I can find alternate synthesis
conditions (for example using n-bromosuccinimide: https://pubs.acs.org/doi/abs/10.1021/jo00208a012?journalCode...) but there really isn’t anything much on the thermal decarboxylation of alpha
amino acids, especially Na salts of such. All I can go off is the general decarboxylation reaction which I’ve outlined multiple times, but that
unfortunately doesn’t include any possible reactions involving the anime or C-N bond, even if none occur.
Since we can’t find any laboratory publications, our best bet will be for one of us to try it. For that to occur though we need to devise a
conclusive test for methylamine that doesn’t involve reaction at the amine. Detection of HCN by MeNH2 decomposition may be one route, but is
exceedingly dangerous if we’re dealing with hundreds-milligram scales.
According to ChemicalBook, MeNH3Cl melts at ~232C whereas NH3Cl melts/decomposes at ~340 so that’s a good test to focus on. Should tell us whether
the major amine produced is methylamine or ammonia, if someone wants to perform the experiment for the amateur community. Won’t take more than
bubbling the pyrolysis products of sodium glycinate/NaOH through hydrochloric acid then collecting the crystals for MPA; I’d try it myself but a),
my clamps are broken so I can’t do a distillation, and b) I don’t have any glycine.
In chemistry, sometimes the solution is the problem.
It’s been a while, but I’m not dead! Updated 7/1/2020. Shout out to Aga, we got along well.
|
|
|
Pumukli
National Hazard
   
Posts: 686
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
I would give it a go.
My plan is roughly: put a few NaOH beads into a mortar, put some glycine powder in and mix (and homogenize, pulverize) them quickly, before they
liquify much. Then put a gram or so from this mixture into a test tube and heat it above a flame (or heat gun) and see what (and how) happens. Then
smell the exit-gas (or find my old Nessler's reagent and test the color change.)
If the thing really reeks of ammonia or an amine then I would try it on a bigger scale in a 100ml flask (or something like that) and would distill off
the amine into cold water.
Then we were back to stage 1: should find a simple, reliable, available route for identification. As I'm mostly into organic chemistry I thought of
acylating the amine with something and determining the properties of the resulted amide. Unfortunately my m.p. meter is non-functioning at the moment
but could be brought back to life in a few days. (needs oil).
|
|
|
LearnedAmateur
National Hazard
   
Posts: 513
Registered: 30-3-2017
Location: Somewhere in the UK
Member Is Offline
Mood: Free Radical
|
|
If you have access to chloroform and potassium hydroxide, the carbylamine reaction is another option for detection. Works with any primary amine, and
should be a prime test in this instance.
“In this reaction, the analyte is heated with alcoholic potassium hydroxide and chloroform. If a primary amine is present, the isocyanide
(carbylamine) is formed which are foul smelling substances.”
R-NH2 + 3 KOH + CHCl3 -> R-(N+)(C-) + 3 KCl + 3 H2O
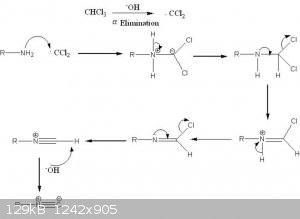
In chemistry, sometimes the solution is the problem.
It’s been a while, but I’m not dead! Updated 7/1/2020. Shout out to Aga, we got along well.
|
|
|
DIBALL
Harmless

Posts: 25
Registered: 4-1-2017
Member Is Offline
Mood: No Mood
|
|
I just remembered, that Ammonium chloride is poorly soluble in ethanol, but Methylamine HCl is soluble. So it should be quite easy to distinct those
two. If the gas is bubbled into HCl and the crystals are soluble in ethanol, it´s Methylamine. Maybe I will give this a try this weekend.
|
|
|
Pumukli
National Hazard
   
Posts: 686
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
That's it, the carbylamine reaction!
I'll go that way. It is quick and can be done theoretically in a test tube.
MeNH2 should produce something stenchy.
NH3 should produce HCN in (alkaline) solution, so it would not have much smell. (And would not be lethal smelling it either.)
According to this:
http://www.sciencemadness.org/talk/viewthread.php?tid=6915
The presence of ammonia could be detected by the prussian blue test (via the formed cyanide) too.
|
|
|
Pumukli
National Hazard
   
Posts: 686
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
Preliminary result:
We all know that preliminaries could have big mistakes, but here it goes anyway.
I put 5-6 NaOH grains into a test tube and with a spatula sprinkled them with some glycine powder. Then applied about 0.5 ml distilled water via an
eye dropper into the tube. I caught the test tube with a smaller diameter flask clamp and heated above open (gas) flame. At first heated along the
full length of the tube to prevent water condensation and reflux and cautiously heated the content at the bottom of the tube last. It quickly boiled
and the glycine dissolved. As the water distilled off some sort of faint purplish discoloration of the melt could be noticed. When I removed the test
tube from the flame and let it cool a bit the liquid melt quickly solidified.
Melted it again and soon started a reaction: the liquid was bubbling like boiling water but at a point the so far clear and translucent liquid became
turbid (milky appearance) and I think started releasing CO2. I base this statement on the different appearance of the bubbles: the whole mass was full
of small gass bubbles and it seemed as if I poured acid on an alkaly carbonate. At the same time I smelled the gasses from the test tube and there was
the unmistakeable amine (or ammonia) stench. So far so good, decarboxylation seems to work.
Quickly cooled the test tube (did not want to loose all the amine/ammonia by overheating, contents quickly crystallized), dropped about 10 drops of
chloroform into the tube and started heating it again. It quickly started to boil but another reaction also took place: the so far milky appearance of
the mixture became yellowish. And the smell of the vapours changed: it definitely got some sort of nauseating stench. In the end the sharp amine
stench disappeared but some sort of disgusting, burnt protein - like smell remained.
I assumed it as positive result for MeNC, although I never smelled that compound before.
I repeated the reaction with a few drops of Roundup as well. (You know, it contains isopropylamine salt of glyfosate.) I was curious how the
carbylamine test would go.
It went like this:
A few drops of Roundup and 3 NaOH grains and a few drops of CHCl3 at the bottom of another test tube. Heated on flame.
At first distinct amine odour in the air. Then some sort of rection ensued: the mixture became turbid, chloroform started refluxing from the walls of
the test tube and an even more disgusting stench developed than in the previous reaction. It was like burnt caramell or burnt coffee mixed with
decomposing meat and lingered long in my throat. (for a few minutes.)
So carbylamine rection seems positive again, but I never smelled iPrNC before. But if we are after malodorous compounds with this test then I'm pretty
sure I got there.
I will test the remnants in test tube No 1 later for cyanide as well.
|
|
|
Chemi Pharma
Hazard to Others
  
Posts: 349
Registered: 5-5-2016
Location: Latin America
Member Is Offline
Mood: Quarantined
|
|
Quote: Originally posted by Pumukli  | Melted it again and soon started a reaction: the liquid was bubbling like boiling water but at a point the so far clear and translucent liquid became
turbid (milky appearance) and I think started releasing CO2. I base this statement on the different appearance of the bubbles: the whole mass was full
of small gass bubbles and it seemed as if I poured acid on an alkaly carbonate. At the same time I smelled the gasses from the test tube and there was
the unmistakeable amine (or ammonia) stench. So far so good, decarboxylation seems to work.
Quickly cooled the test tube (did not want to loose all the amine/ammonia by overheating, contents quickly crystallized), dropped about 10 drops of
chloroform into the tube and started heating it again. It quickly started to boil but another reaction also took place: the so far milky appearance of
the mixture became yellowish. And the smell of the vapours changed: it definitely got some sort of nauseating stench. In the end the sharp amine
stench disappeared but some sort of disgusting, burnt protein - like smell remained.
I assumed it as positive result for MeNC, although I never smelled that compound before. |
Well done @Pumukli, but I have to make some observations:
I- When you say CO2 are released from the test tube, may be the gas were methane (CH4), in my theory.
II - If the gas evoluted were CO2, it will react in situ with NaOH to form a carbonate and water. No bubbles would be visible. Think about it.
III - although methylamine are inflamable and ammonia not, in this case the test of an open flame wont work cause, in your theory CO2 and CH3NH2 will
be released and this mixture will burn at a flame. In my theory NH3 and CH4 will be released and this mixture will be inflamable too, cause the
methane.
IV - The carbylamine test isn't enough to distinguish NH3 and CH3NH2. It's a proper test for distinguish primary from secondary and terciary amines.
But think about it. Ammonia is a kind of primary amine eihter and will form a isocyanide too with chloroform (hydrogen isocyanide), while CH3CH2 will
form methyl isocyanide. According wikipedia, both have a desagreeable smell and the olfact couldn't distinguish one from another.
V - I agree with @Diball when he said the better test to distinguish one from another is the ethyl alcohol solubility of the chloride or chloridrate.
This question have been already discussed here at sciencemadness. See this thread:
http://www.sciencemadness.org/talk/viewthread.php?tid=11293&...
|
|
|
Pumukli
National Hazard
   
Posts: 686
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
Since then I tried carbylamine test on NH4Cl. I could only smell sharp ammonia stench, nothing rotting/burnt meat.
I also tried checking for cyanide in the water solutions of the remnants. (Acidification either by citric acid/hydrochloric acid then addition of
FeSO4 solution.) It was negative in all cases. I mean I did not get prussian blue in any case. What I got is a clear yellow solution in case of NH4Cl
and a blackish/browish precipitate from the amines/isocyanides which precipitate quickly dissolved in the excess of acidic reagent, giving the same
clear solution as did NH4Cl.
Now I have to do the washing because I can't put a needle on my lab bench. I need tydiing up before going any further. :-)
(Unfortunately I don't have any cyanide handy to make a definite positive prussian blue test.)
Btw bubbling/CO2 evolution:
You are right, in the excess of alkali no CO2 bubbling should be noticeable! Oh my! :-) I correct myself: what I did observe was NOT CO2 evolution but
either NH3 or MeNH2!
When I tried the test on NH4Cl it gave a very similar "fizzing" as NaOH freed the NH3. It bubbled like freshly opened soda water.
[Edited on 17-3-2018 by Pumukli]
|
|
|
Pumukli
National Hazard
   
Posts: 686
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
Making methylamine from glycine
testing a hypothetical pathway
After the promising results of the preliminary tests I decided I'd do the decarboxylation on a bigger scale.
Into a 100 ml roundbottom flask I measured 20 g (0.266 mol) glycine. I dissolved 12 g NaOH (0.3 mol) in roughly 26-28 ml water in a 100 ml beaker with
external cold water bath cooling. Poured the solution into the flask, dropped in two boiling stone pieces too.

Then attached a distilling cooler onto the flask with thermometer. Attached an „auxiliary collector” - a 50 ml pear shaped flask- to the end of
the cooler. Attached a teflon tube to the vacuum-port of the cooler and put a glass filter tulip to the other end of the tube. The real collector was
a 150 ml beaker with 52 g water and a teflon stir bar sitting in a plastic box of cold water on a magnetic stirrer. (It is a bit complicated to
explain, especially when I try to translate in a hurry, but you will see on the photos what I'm talking about. This is my tried and reliably working
setup for amine distillations.)
 
I put the distilling flask into a sand bath on a hotplate and started to distill off the water.
The temperature of the sand was around 325 C by the time the water started to distill. The cooler was cooled by air (aquarium air pump) and wet tissue
papers placed on and around its body. At first it was a bit hard to condense effectively the water but later when the mixture became drier the cooling
became easier.
After I distillied off some water I detached the auxiliary collector and measured the weight of collected water. (I repeated this measurement several
times during the distillation.) After I collected more than 10 millilitres of water I put one drop of phenolphtalein into the auxiliary collector. It
turned pink immediately. It was a bit surprising because I could not smell any ammonia or amine at this point. Maybe the fine NaOH mist from the
bursting bubbles was washed down with the condensing water.

After 25 millilitres of water was distilled the appearance of the contents in the distilling flask changed. So far it was absolutely clear and
translucent, at this point it became almost foamy as it boiled. The cooling became easier because less water was distilling now. Meanwhile the
temperature of the sand bath reached 333 C.
At 27 millilitres water collected there was still no amine or ammonia, not even traces. There was some sort of faint and unpleasant (burnt?) smell but
nothing serious. Sandbath at 341 C.
Around this point I changed the thermometer in the sand bath from the mercury/glass one to a type-K thermocouple/digital meter because the mercury
thermometer was approaching the end of its scale (360 C). Of course it made all the previous readings uncomparable to the new ones. The new
thermometer read 210 C at the spot in the sandbath where I could insert it in. :-) And around these crucial moments the power controller of my
hotplate died! Now on I could only On/Off switch it at full throttle. :-) At 1300 W available power it is not easy to smoothly do it.
I forgot to turn it off for a few more seconds and the hot plate was glowing in red! :-) Fortunately the sand had substantial thermal inertia and the
heating up was steady and smooth according to the thermometer.
By the time the sand bath reached 233 C (new meter!) I could collect 28 milliliters of water and the contents in the distilling flask became fairly
dry. Almost no water was condensing back. Neither any amine smell could be detected to my surprise!
Around 250 C sand bath temperature I realized that something is collecting on the inside wall of the distilling flask. There were „water” droplets
on the flask wall – but at this temperature they can't be water, they would boil up explosively! But no, these droplets just refluxed back from the
wall and flew down in the mixture with almost no boiling. Of course it was not the hoped amine either!

I have to mention that NO apparent bubbles could be seen so far in the real collector during the distillation. I can safely say that no uncondensable
ammonia/methylamine/methane/whatnot appeared in the gas so far! On the other hand the vapour thermometer registered exactly 100 C so far. Not more not
less, exactly 100 C since the water started distilling.
At around 273 C sand bath temperature something changed in the flask: its content got a definite yellowish hue. At the same time something started to
bubble into the real collector! I smelled the off gas and it was surely amin/ammonia! Now it is here! :-) The vapour thermometer quicly declined,
registered 82 C in these moments.
And the not so exact thermal control ruined everything in the coming two minutes... Some sort of exotherm occured and filled with an interesting brick
red and yellow foam the distilling flask. The foam started to spew off smoke and yellow liquid condensed in the auxiliary condenser (a few
millilitres, maybe 5 or less). I turned off the heat, removed some sand from the sand bath to help cooling but it was too late. Tomorrow I will have
to do the washing up again. :-)
I doubt this dry distillation could be tamed to a productive synthesis. It did produce some sort of amine, maybe only ammonia. Unfortunately the point
of amine production and runaway is too close to one another. Tomorrow I'll look after the remnants and decide if I could do anything meaningful with
the condensed products both in the auxiliary and the real collectors.
I may try it again with a reliably working thermal controller one day. Maybe that way one could balance on the fine line between amine/ammonia
production and thermal runaway, though I'm a bit sceptical.
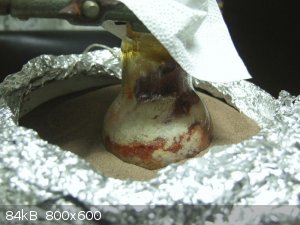 
[Edited on 17-3-2018 by Pumukli]
|
|
|
Chemi Pharma
Hazard to Others
  
Posts: 349
Registered: 5-5-2016
Location: Latin America
Member Is Offline
Mood: Quarantined
|
|
Nice pictures @Pumukli. Sorry about the frustration with your experiment.
I suggest you try again and after the water were all distilled, you substitute the cooler by a silicone hose before the reaction mix runaway start up
with amine/ammonia evolution and bubble the gas into a flask with HCl in ethyl alcohol solution.
I think you can do it even with your broked hot plate, since you're using a sandbath. Just monitoring the temperature and take note to publish here.
If you got a white solid precipitated, even a tiny amount, the gas were ammonia and the ppt ammonium chloride. If nothing precipitates the gas were
methylamine and the hydrochloride will remain in the solution.
|
|
|
Pumukli
National Hazard
   
Posts: 686
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
ChemiPharma:
I use this complicated setup because I learned (of course the hard way) that the tubing (silicone or not) what I can buy here is attacked by the
distilling amine vapours in a few minutes. It discolorates first then starts to release some sort of oily substance and contaminates the distilling
amine. Hence the all-glass vapour path and the PTFE tube at the end. This way the distilled amine remains pure.
I'd rather avoid the runaway reaction at all cost. It makes a very contaminated end-product (what you can see in the last picture, that is thick
white smoke in the final collector and filter tulip.) I suspect that in a runaway there would be all sort of crap and probably a complex mixture of
various N-compounds which would make identification even more troublesome.
Very careful and slow heating MIGHT make possible to distill something fairly pure off of the mixture. For this I have to repair the power controller
at first and have a full day free time to really slowly approach the sensitive thermal region.
Edit: duplicated pictures removed.
[Edited on 18-3-2018 by Pumukli]
|
|
|
EilOr
Harmless

Posts: 20
Registered: 12-12-2017
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by DIBALL  | Well in theory it should be possible, or not?
I tried it with 1g of Glycine in Propylenglycol (not really soluble when cold) and 1g of Cyclohexanone and refluxed it for an hour...
|
As you've used such a high amount of catalyst you may have made the methylamine-cyclohexanone imine as a main product
Also I think PG, despite the high bp., is a very suboptimal solvent as it's too polar.
I would try to use Mentha spicata var.crispa essential oil (OTC spearmint oil, consists mostly of carvone) as in my experience it's one of the best
catalysts for decarboxylation, even better than Cyclohexenone and a bottle can be had for 3-10€.
As solvent I would use good old genuine terpentuine oil as it has optimal boiling point, is known to be very high yielding at least with aromatic
amino-acids, quite cheap and OTC, but Shellsol T (a good less smelling terpuintine-substitute for many reactions) and especially DMSO or DMF etc.
should also work
I wonder if methylammoniumcarbonate would be a side product or maybe even the main-product, causing low yields.
Maybe you could use an aqueus copper solution to detect methylamine (forming colorful complexes)
[Edited on 18-3-2018 by EilOr]
|
|
|
DIBALL
Harmless

Posts: 25
Registered: 4-1-2017
Member Is Offline
Mood: No Mood
|
|
Great job and pictures Pumukli! That brings us a good step closer!
I retried this a couple days ago:
5g of glycine and 10g of NaOH were dissolved in way too much water (40mL) and the water was distilled off in a heating mantle. A hose was attached,
which lead to a flak, which was filled with a 15% HCl solution. When the water was driven off, the now solid NaOH/glycine mixture started to melt and
very soon after, a rapid reaction occurred! While during the water distillation only a couple bubbles reached the HCl solution, now it was a
vigorously bubbling. The NaOH/glycine solution became really foamy and yellowish and I had to take the flask off the heating mantle, because it was
starting to get into the hose. After cooling, I evaporated off the HCl solution and tried to dissolve the remaining ammonium/amine chloride in acetone
(ammonium chloride is soluble in it, methylamine not) but it didn´t dissolve. After filtering I added anhyd. ethanol and it dissolved quite well
(which methylamine does, but ammonium chloride not). Then I recrystallized it.
Looks for me like it is indeed methylamine! Only thing that bothers me, is, that the crystals didn´t look like the ones of the methylamine I had
before. Also it isn´t really hygroscopic. Well when I have my new thermometer I will determine the melting point and then we will see for sure!
Maybe we could try with Trimethylglycine as well, since it should be easier to tell apart from the ammonia.
@Melgar and EilOr
Indeed I have mixed it up with CyclohexEnone... If the other method doesn´t work, I will try it with peppermint oil.
|
|
|
zed
International Hazard
    
Posts: 2277
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Ummm. Yeah. I've run this reaction, and it seems to work, sort of.
First. If you attempt to run this de-carboxylation, neat, on a bulk amount of glycine, it will fail. You will produce massive amounts of brown
polymeric crud.
If you disperse finely divided glycine, in a large volume of clean sand, then slowly distill the mixture, you will accumulate a reasonable yield of
what appears to be Methylamine/Methylamine Carbonate. Smells right, looks right.... But, don't trust it. Convert it to the hydrochloride, and
recrystallize it, ala Vogel. The idea is to isolate individual molecules, via dispersion in sand... so they cannot auto-condense into crud. Though
the product is colorless, it appears that it is a mixture, not pure Methylamine. Might be anything in there.
Myself, and my colleague, Dr. CrazyFingers, once ran this reaction fairly successfully. Crazyfingers, impetuous boy, ignored my warnings and
attempted to use this material without further purification. Well, garbage in....Garbage out.
As designed, our reaction was more-or-less a pyrolysis. Just a distillation flask, full of sand and Glycine, in an old electric stove, with a hole
cut in the side, to allow the side arm to exit.
As I said, it seemed to work fairly well, but a real workup would be required to confirm. At the time, I had other priorities.
|
|
|
LearnedAmateur
National Hazard
   
Posts: 513
Registered: 30-3-2017
Location: Somewhere in the UK
Member Is Offline
Mood: Free Radical
|
|
Didn’t think of that, probably because I haven’t tried it out, very useful info for anyone attempting this. Could you also use other refractory
materials like alumina and magnesia to good success or is silica the best/only option? Just requesting info for others in the case that the
aforementioned is on hand.
In chemistry, sometimes the solution is the problem.
It’s been a while, but I’m not dead! Updated 7/1/2020. Shout out to Aga, we got along well.
|
|
|
| Pages:
1
2
3 |