| Pages:
1
..
13
14
15
16
17
..
24 |
snooby
Hazard to Self
 
Posts: 88
Registered: 24-5-2013
Member Is Offline
Mood: No Mood
|
|
Thanks for noticing the '' A spontaneous explosion of sodium nitrotetrazole dihydrate during drying has been reported. See page 8 of the attached
document ''. I was not able to find it anymore in the shitload of patents that are written about nitrotetrazoles, but it's something to keep in mind!
Like I said I will try the DBX-1 and post results
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Snooby, from that article:
"In the light of this incident, doubt must be cast on the stability of NaNT.2H20 although it should be stressed that we have prepared many batches in
the past without any similar problems. A further batch of NaNT.2H20 was subsequently prepared without incident."
This looks like a one-time thing. I have prepared NaNT.2H20 many times and never with any spontaneous explosions. Considering the authors mention it
was exposed to zinc/acid spray, a lot more could be going on here than simple NaNTX.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Maybe it only happens once in a thousand times, it just somehow goes wrong.
Statistically, then there is exactly the same chance of the anomaly if this is the
first of a series of one thousand experiments, or it is the next experiment following 999 which have gone before without incident. So there should be
remembered that there are three kinds of lies; there are lies, damn lies, and statistics.  Murphy's law may not be the unvarying and inviolate supreme law of the land or universe, but too often it does seem close enough for
government work. Murphy's law may not be the unvarying and inviolate supreme law of the land or universe, but too often it does seem close enough for
government work.
That gel formation intermediate reported during the formation of DBX-1 is possibly analogous to the remarkable effect that also occurs for lead
styphnate under very specific conditions.
http://www.sciencemadness.org/talk/viewthread.php?tid=25035&...
http://www.sciencemadness.org/talk/viewthread.php?tid=2827&a...
With regards to my post yesterday being edited,
http://www.sciencemadness.org/talk/viewthread.php?tid=8144&a...
my apology to snooby for our interrupted discussion.
I apologize for the temporal disconnect and temporary constraint placed upon discussion of quoted material not yet fully approved for publication.
I think an earlier post which I made some years ago may have usefulness in the reaction sequence algebra being applied to development of DBX-1
http://www.sciencemadness.org/talk/viewthread.php?tid=8144&a...
However, I am not sure how useful that idea could be for not having tried it, converting the first obtained acid copper salt gotten via Sandmeyer to a
neutral mixed sodium copper salt. I never contemplated this idea as anything potentially useful except as a shortcut that may produce a more useful
product directly with little additional manipulation, or as a filtering aid for the isolated material which would then be subjected to similar further
workup, being digested with sodium hydroxide, but requiring less base for complete conversion to the sodium salt, than would the acid copper salt
ordinarily gotten from the Sandmeyer reaction unmodified, or not subsequently further reacted in the same pot.
(Na)2[CuII(NT)4(H2O)2] may possibly be convertible to DBX-1 by sequential reaction with AcOH and/or HCl followed by subsequent treatment with a
mixture of Cu(OAc)2 and Na ascorbate.
[Edited on 16-11-2013 by Rosco Bodine]
|
|
|
snooby
Hazard to Self
 
Posts: 88
Registered: 24-5-2013
Member Is Offline
Mood: No Mood
|
|
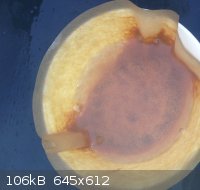
DBX
Did some homework 2day. This stuff should be DBX-1. I have used the synthesis without NaNTZ but only acid CuNTZ and sodium-ascorbate. I cannot post
the synthesis again because it is patented at the moment.
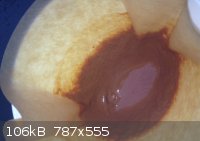
[Edited on 17-11-2013 by snooby]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Earlier in the thread was mentioned a reference by Glligan and Kamlet that was an inaccesible paper. Attached is that file.
Also is an interesting microprocess continuous reaction scheme which gets around the need for a Sandmeyer reaction and produces sodium nitrotetrazole
directly.
Attachment: Gilligan and Kamlet 1976 research ADA036086.pdf (1.2MB)
This file has been downloaded 1032 times
Attachment: US7253288 Continuous microreactor process for sodium nitrotetrazole.pdf (1.2MB)
This file has been downloaded 1142 times
[Edited on 18-11-2013 by Rosco Bodine]
|
|
|
snooby
Hazard to Self
 
Posts: 88
Registered: 24-5-2013
Member Is Offline
Mood: No Mood
|
|
Interesting! In the article continuous microreactor.... they mention a synthesis for pure NaNTZ which should be even more safe and easy.. why all the
effort for NaNTZ with purifying etc in other articles 
@ Rosco: this DBX looks like the right color right? Think I made a little to much at once. Is it advised to separate the stuff into 0.5 grams portions
or even less? Don't really know what to expect of it when it's dry!
[Edited on 18-11-2013 by snooby]

|
|
|
snooby
Hazard to Self
 
Posts: 88
Registered: 24-5-2013
Member Is Offline
Mood: No Mood
|
|
As promised. In the middle is the DBX-1. However it is still drying! Think it is almost totally dry. How much of the stuff will set of PETN? I red 40
mg did the job but I am not sure anymore.

|
|
|
snooby
Hazard to Self
 
Posts: 88
Registered: 24-5-2013
Member Is Offline
Mood: No Mood
|
|
I managed to make 2.8 gram of pure, extracted NaNTZ! It is slightly yellow colored. Should I be concerned that the stuff is going to dehydrate? What
is the best way to store the stuff in your opinion?
Same for the AgNTZ, can it be stored under some distilled water or something else?
The DBX-1 seems to be dry, though it is still not as reactive as for example AgNTZ. Quite sensitive towards impact though..
[Edited on 23-11-2013 by snooby]
|
|
|
Dany
Hazard to Others
  
Posts: 482
Registered: 3-8-2013
Member Is Offline
Mood: No Mood
|
|
generally, if the product crystallize with water molecule it is difficult to dehydrated. When water molecule crystallize with your product it is now a
part of this substance and not like a wetted substance that will dry with time. A simple example is crystalline CuSO4.5H2O which
stay hydrated at room temperature. dehydration occur at elevated temperature. So maybe if you heat your synthesized EM to hundred of degrees you will
dehydrate it (with probably a decomposition). of cours this is not the case since you will store your EM at room temperature.
Dany.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
recent patent applications
US20130317232 MTX-1 Tetrazene Derivative Having Improved Stability
US20130204005 DBX-1 related patent
Attachment: US20130317232 Alternative to Tetrazene.pdf (748kB)
This file has been downloaded 1122 times
Attachment: US20130204005 Lead Free Primary Explosive.pdf (1.5MB)
This file has been downloaded 998 times
|
|
|
Ragnar
Harmless

Posts: 6
Registered: 7-10-2013
Member Is Offline
Mood: No Mood
|
|
I would have expected the DBX-1 you synthesized to be a little more redish. I would not keep for long term storage. However, I doubt that you would.
Any excess can be rendered inert by combing it with 10% sodiumhydroxide. This is an extremely interesting compound and is being sought as a
replacement for Lead Azide for military applications. Based on former life, I have learned that the military is always trying to find ways to be more
earth friendly. They don't care about what they blow up, but heaven forbid if there are heavy metals left behind.
|
|
|
snooby
Hazard to Self
 
Posts: 88
Registered: 24-5-2013
Member Is Offline
Mood: No Mood
|
|
The DBX-1 turned almost black in the end. When dry it became little chunks of DBX-1 who where impossible to be powdered. They were quite sensitive for
impact but did not work when ignited by fuse unfortunately. The synth I used was whithout the use of NaNTZ. Maybe this synth was not so good after
all. I now have an amount of cupric(II)cloride and I will try the synth again in a while. I indeed decided to put away the stuff. Have you tried DBX-1
yourself btw?
|
|
|
snooby
Hazard to Self
 
Posts: 88
Registered: 24-5-2013
Member Is Offline
Mood: No Mood
|
|
Btw im trying on BNCP. But first I want to do the synth of mono-(5-nitro-H-tetrazolato-N)triammine copper(II) perchlorate (MNCuP). In the synthesis
Engager says:
'To solution of 40 ml 70% HClO4 in 100 ml of water, 3.5g (0.01379 mol) of carbonato triammine copper(II) nitrate in 30 ml of water is added with
stirring. Deep blue color of copper carbonate complex quickly turns to transparent sky blue. Resulted mixture is heated to 60 ± 10С and left to
stand at this temperature for half of hour and heated to ~80C, then concentrated solution of sodium 5-nitrotetrazolate (0.00689 mol) is added by drops
with stirring'.
I have no idea how much (0.00689 mol) of sodium 5-nitrotetrazolate is! Don't know the moll mass of the stuff. Anyone got an idea?
|
|
|
Dornier 335A
Hazard to Others
  
Posts: 231
Registered: 10-5-2013
Location: Northern Europe
Member Is Offline
Mood: No Mood
|
|
Sodium 5-nitrotetrazolate has the formula NaCN5O2, so the molar mass is 137.033 g/mol. (dihydrate: 173.063 g/mol)
|
|
|
snooby
Hazard to Self
 
Posts: 88
Registered: 24-5-2013
Member Is Offline
Mood: No Mood
|
|
Thanks for information.
100% / 1 mol = 100*0.00689 = 0.689
173/100 = 1.73*0.689 = only 1.191 grams of NaNTZ for every 3.5 grams of carbonato triammine copper(II) nitrate. Seems to be a little less for that
amount of HCLO4 also
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
High energy tetrazole-based compounds as lead replacement explosives
www.chemikinternational.com/year-2013/year-2013-issue-1/high-energy-tetrazole-based-compounds-as-lead-replacement-explosives-wojewodka-a-witkowski-
t
That article here _
Attachment: High energy tetrazole-based compounds as lead replacement explosives.zip (840kB)
This file has been downloaded 770 times
Techniques to Disrupt, Deviate and Seize Control of
an Internet Forum In case you wonder W T F ! is going on here
?
www.zerohedge.com/contributed/2012-10-28/cointelpro-techniques-dilution-misdirection-and-control-internet-forum https://web.archive.org/web/20120814124000/www.washingtonsblog.com/2012/08/the-15-rules-of-internet-disinformation.html
___________________________________________________________________________________________________________________
___________________________________________________________________________________________________________________
___________________________________________________________________________________________________________________
|
|
|
snooby
Hazard to Self
 
Posts: 88
Registered: 24-5-2013
Member Is Offline
Mood: No Mood
|
|
Soooo ppl! Hello again. I have great news! I have made (in steps og 1 grams) superpure NaNTZ! About 7, dissolved in deminiarlised water. (what should
the PH be btw?P
I want BNCP: I got sodium perchlorate and coblat sulfate , ammoniumcarbonate and H202 30%!
BUT i only got 5% ammonia.. insead of 25.
So I got everyting except 25% ammonia..My question, can i use Household ammonia and do it like this: inseatd of 250 ml 25% ammonia i will use 1150 ml
5% ammonia. And then put 14 ml h202 30% and then eveporate till 300 ml. Whil this work ?
500 ml H2O + 250 ml 25% ammonia solution. Resulted dark violet solution is oxidised by addition of 14 ml of 30% hydrogen peroxide. Solution is
allowed to sit for 30 minutes, after that period it is placed on boiling water bath and is evaporated to 300 ml volume.
1. Preparation of [Co(NH3)4CO3]2SO4*3H2O complex. 47g CoSO4*7H2O is dissolved it 125 ml of water and is added to solution of 100g (NH4)2CO3 in 500 ml
H2O + 250 ml 25% ammonia solution. Resulted dark violet solution is oxidised by addition of 14 ml of 30% hydrogen peroxide. Solution is allowed to sit
for 30 minutes, after that period it is placed on boiling water bath and is evaporated to 300 ml volume. During course of the concentration process,
solid ammonium carbonate (25 g) was added in installments. Solution is filtered from unsignificant ammount of precipitated black cobalt oxide and is
further concentrated to 200 ml volume. Solution is slowly cooled to room temperature, product precipitates as small deep-red prisms. They are filtered
out from the solution and allowed to dry at room temperature. Yield is 16 g of pure product, mother liquer can be further concentated with addition of
ammonium carbonate to get more 16 g of complex, but it is less pure.
2. Preparation of [Co(NH3)4CO3](ClO4) complex. 16g of complexed synthesised on first stege is dissolved in 320 ml of water. Solution is filtered and
16g of sodium perchlorate in 40 ml of water is added with stirring. Mixture is cooled on ice (or in freezer) for 3-4 hours. Product precipitates as
small, lustrous, sharp violet prisms. Crystalls are filtered off, washed with small ammount of ice cold water and dried at room temperature. Yield is
about 14 gramms.
3. Preparation of BNCP. 14g [Co(NH3)4CO3](ClO4) is dissolved in 140 ml 10% perchloric acid, and solution of 26.5g of sodium 5-nitrotetrazolate
dihydrate in 450 ml of water is added with stirring. Solution is placed on boiling water bath, and allowed to sit there for 4 hours. Solution is
slowly cooled to room temperature and then cooled in freezer to 10°С, precipitated crystalls of BNCP are filtered of, washed with cold water,
and recrystallised from 1% perchloric acid. Yield is 12.9g.
Photos of reaction products: first is tetraminocarbonato cobalt (III) sulphate trihydrate (product 1), second is tetraminocarbonato cobalt (III)
perchlorate (product 2), third is crystalls of BNCP.
|
|
|
snooby
Hazard to Self
 
Posts: 88
Registered: 24-5-2013
Member Is Offline
Mood: No Mood
|
|
I managed to make some very nice Orange Red Crystals of BNCP. When ignited by a flam the result is flashy cracklings! I saw a video of engager who was
able to use it in a detonator.
Is it nessecary to grind the BNCP fermly before using it!? It is not was sensitive toward flame as I was hoping.
|
|
|
snooby
Hazard to Self
 
Posts: 88
Registered: 24-5-2013
Member Is Offline
Mood: No Mood
|
|
Ok I was succesful in detonating BNCP in a plastic tube! But, sensitivity for friction should be low.. but with hammer scratching causes detonation
rather easy (3.2KG should be the standard). For PETN this is 6 kg, but I dont get it going how hard I try. Is this normal for BNCP? My advise for
BNCP, still stay safe with it...
The test was as follow: I took a sample of BNCP on a brick, very sandy and irregular surface. Then scratching with hammer with some pressure. Almost
immediately detonation.
|
|
|
Dornier 335A
Hazard to Others
  
Posts: 231
Registered: 10-5-2013
Location: Northern Europe
Member Is Offline
Mood: No Mood
|
|
Using a surface like a brick or concrete drastically decreases the weight needed for in impact and friction tests. My nano-Armstrong's mix is very
difficult to set off steel against steel, much easier with steel against concrete.
|
|
|
snooby
Hazard to Self
 
Posts: 88
Registered: 24-5-2013
Member Is Offline
Mood: No Mood
|
|
I thought BNCP was the BEST and safest primer nowadays. Is it no surprise I can detonate it with en moderate hamerstrike? It is like 3 times less
senstive then AgNTZ for example. But still, it is farrr more senstive then PETN!
|
|
|
snooby
Hazard to Self
 
Posts: 88
Registered: 24-5-2013
Member Is Offline
Mood: No Mood
|
|
This is BNCP detonating aboout 30 mg!
Attachment: bncp video.MOV (1.9MB)
This file has been downloaded 1041 times
Pure BNCP and detonator picture

[Edited on 17-4-2014 by snooby]
[Edited on 17-4-2014 by snooby]

|
|
|
snooby
Hazard to Self
 
Posts: 88
Registered: 24-5-2013
Member Is Offline
Mood: No Mood
|
|
synth of mono-(5-nitro-H-tetrazolato-N)triammine copper(II) perchlorate (MNCuP). In the synthesis Engager says:
'To solution of 40 ml 70% HClO4 in 100 ml of water, 3.5g (0.01379 mol) of carbonato triammine copper(II) nitrate in 30 ml of water is added with
stirring. Deep blue color of copper carbonate complex quickly turns to transparent sky blue. Resulted mixture is heated to 60 ± 10С and left to
stand at this temperature for half of hour and heated to ~80C, then concentrated solution of sodium 5-nitrotetrazolate (0.00689 mol) is added by drops
with stirring'.
So: Sodium 5-nitrotetrazolate has the formula NaCN5O2, so the molar mass is 137.033 g/mol. (dihydrate: 173.063 g/mol)
So I calculated:
100% / 1 mol = 100*0.00689 = 0.689
173/100 = 1.73*0.689 = only 1.191 grams of NaNTZ for every 3.5 grams of carbonato triammine copper(II) nitrate. Seems to be a little less for that
amount of HCLO4 also.
Is this calculation correct?? Because in the synth of BCNP, you need to add 2.6 grams of NANTZ for every 1.4 grams of cobaltperchlorate stuff! So I
think 1.191 grams NANTZ is very litle for 3.5 grams carbonato triammine copper(II) nitrate.
So is this correct, or am I making a mistake?
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
You are all welcome....OTC aminoguanidine 
http://www.worldantiagingstore.com/brands/profound-products/...
http://www.supersmart.com/en--Blood-Sugar-Glycation--Aminogu...
[Edited on 24-4-14 by The_Davster]
|
|
|
Microtek
National Hazard
   
Posts: 845
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
30 dollars for 7.5 grams... A little steep.
|
|
|
| Pages:
1
..
13
14
15
16
17
..
24 |