| Pages:
1
2 |
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Large Carboxylic acid "construction"
I've read through the entire alkane section of a thick organic chem book I took out of the library and it hasn't helped me. The internet is clouded
with garbage, so I have come asking here. I am looking to make 8-methyl,nonanoic acid for use in a later synthesis. Any brillant ideas on how to
produce the stuff? I don't really need a lot, few grams maybe. I was thinking the n-heptane I have might be of use, possibly hitching it to an
isopropyl group (I assume grignards come into play here, therefore the heptane would need to be made into a 1-haloheptane, but the issue of
selective oxidation comes into play afterwards (as well as how to make the haloalkane in the first place)).
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
One, non-trivial route would be:
Cheers,
O3

-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Very nice. My eventual synth target was dihydrocapsaicin since that seemed easier than capsaicin, but this gives me a better idea, since I'd rather
make capsaicin itself. If I start with 5-bromopentan-1-ol, use MEK instead of acetone, and skip the hydrogenation, that should give the "tail" needed
to latch onto vanillin (acyl halide I suppose) after the vanillin has been condensed with hydroxylamine and reduced (or another reductive amination).
Where would I get my hands on some 5-bromopentan-1-ol though? I assume I have to make it since I get 18 hits from google with just that chemical
name, 88 for 5-bromopentanol. 
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
That OH on the alcohol in step 1 is going to trash the Grignard with its acidic hydrogen. I think MOM, MEM, BOM, or THP will work for that.
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Stoicheometric treatment of pentanediol with NaBr/H2SO4 (or THF, polar-aprotic solvent)? Yields would not be great, but it would be cheap and the
product could be had via careful distillation.
it could go like this:
Cheers,
O3
[Edited on 1-5-2007 by Ozone]

-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Sheet! Not Important has a point (overlapping posts, grr). Quick protection with dihydropyran might do the trick.
Whoops!
O3
[Edited on 1-5-2007 by Ozone]

-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
There are some suppliers for 5-bromopentanol though relatively few and none of them is the usual sigma/acros/aesar/etc big corporative one. It can
however be prepared from tetrahydropyran and conc. HBr.
Ozone, acetone can not be used as a solvent for H2SO4 as it self condenses to some red goo in its presence. The dehydration with phosphoric acid
yields two prevalently trans regioisomers (and not only one as you depicted), not to mention that it is unlikely it would go smoth. Chromic acid
oxidizes double bonds. And your THP protected 5-bromopentanol above is not the acetal it should be (the alkoxy side chain must be atached to the
position 2).
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
O3, great diagrams and quick response 
And thanks for pointing out my obvious lack of experience with grignards. 
Gonna be real interesting when I can accumulate all the reagents for this.
Thanks again!
-Eric
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Thanks for the errata, Nicodem!
I fixed the DHP. Acetone/H2SO4 gives mesitylene and tar. At reflux the dehydration should give the thermodynamic products which are both trans (6 or
7-en, there might also be some rearrangement via alkyl migration to give the most stable carbocation)... Chromic acid by itself, when dilute in
acetone (Jone's reagent) is not known for aggressively attacking double bonds.
The preparation of 5-bromopentanol from tetrahydropyran is a very good call (I wish I'd have thought of it! I'll post the papers I found later).
Got to go now, thanks,
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Not to try suggesting of substituting the Grignard reaction with the equally tedious Wittig, but just as an alternative here is another possibility.
However this one starting with epsilon-caprolactone is inappropriate for preparing (E)-8-methylnon-6-enoic acid since the Wittig reaction gives almost
exclusively the (Z) isomer, but it is still good enough for the original question of preparing 8-methylnonanoic acid (for dihydrocapsaicin or whatever
it is meant for).
As for the most appropriate substrate for a Grignard route, perhaps it would be better to start with delta-valerolactone and heating it with
2-amino-2-methylpropanol to obtain the 5-hydroxypentanoic acid equivalent with the COOH protected (the 4,4-dimethyloxazolyl protection is said to be
safe for Grignard reactions). The alcohol can be then brominated with PBr3 and the product used for preparing the organomagnesium intermediate. The
oxazolyl protection can be removed at the end by acidic hydrolysis.
PS: It is certainly too much work for something that can be obtained by hydrolysis of capsaicin, itself isolable from natural sources where it exists
in good enough concentration.

…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Well, I know I could just isolate the natural substance, but I really wanted to synthesize it. I don't have a further use since I wouldn't really
consider eating anything I synthesized. In fresh red savina habenero (hottest pepper out there), capsaicin should exist as roughly 3% of the pepper's
mass, but attempting to isolate it results in a thick pasty, red mix that the industry would call hot pepper oleoresin. I'm sure it could be refined
further, but where's the fun in that? Especially since the final product is a concentrated version of pepper spray canisters (thinned with a solvent
since the capsaicin would be a solid). After the final coupling I'd need to don some strong gloves and wash everything with lots of alcohol
afterwards. Capsaicin is the anti-menthol and just getting it on your skin can cause burns (actually your body just thinks it's being burned, but the
stinging and swelling are the same). My uncle (a bigger fan of hot food than me) once forgot to wash his hands after slicing habanero peppers, used
the bathroom, and never made the same mistake again. 
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Another potential route just to make things more simple by avoiding Grignards and Witttigs:

|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Synthesis of an inconvenient alkene
I've had no success figuring out a good way to make this (deceptively simple) compound without resorting to alkyllithiums (or similar powerful base)
or chromatography of the products. If necessary, a grignard reaction is something I could do, but would rather avoid. The intent is to couple it with
vanillylamine (easily prepared from vanillin) using this procedure: http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=v81...
The final product is capsaicin, which is what makes hot peppers spicy. Thanks for any help you can provide.
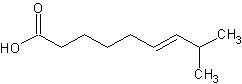
[Edited on 3-23-09 by UnintentionalChaos]
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Off the top of my head, the route I see is from pimelic acid and isobutylaldehyde, through this
http://www.organic-chemistry.org/abstracts/literature/928.sh...
followed by decarboxylation.
The dibromoacid would be substitution on the half ester of the acid, pimelic is made from salicyic acid via sodium reduction in C5 or C6 alcohol
http://www.orgsyn.org/orgsyn/pdfs/CV2P0531.pdf
All in all a rather bothersome route.
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
I've looked into the reactions of CrCl2 with gem-dihalides before. A prime example is the takai olefination (which proceeds by an identical mechanism,
but with a halide replacing the ester funstionality). The 6eq of CrCl2 can also be provided by 6eq of CrCl3 and 8eq or so of Zn dust. This makes the
reactions even more bothersome.
On an unrelated and useless sidenote, pimelic acid would be more easily obtained by ring-opening of caprolactone with a cyanide salt, followed by
hydrolysis, as in: http://www.orgsyn.org/orgsyn/prep.asp?prep=cv4p0496
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Well, Reformatsky on the alpha chloride/bromide plus isobytalaldehyde, then dehydration, the decarboxylation, might also work. Question are the ratios
of double bonds in right and wrong places, and stereochemistry of the double bond.
I can see a couple of others maybes, but need to doodle a bit. Have you any possibilities?
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
Would you consider a relay synthesis from capsaicin? Heh, almost cheating, but I don't know what your goal is.
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
You mean hydrolyze capsaicin to get the acid....out of the question. There are several capsaicinoids in peppers which would be infuriating to separate
(the tails are what is different). If I wanted to spend a year doing chromatography, I would do that and just keep the pure capsaicin.
not_important- the dehydration is a huge problem. It's going to mainly form the E-alkene, but will preferably dehydrate to the more substituted double
bond, aka. the one I don't want. I've been through a lot of options in my head and I don't see anything good, hence me posting here. I need a group to
temporarily make the terminal bit quaternary. That will force the dehydration to go where I want and then the group can be removed, but I dont know
what group or how to put it there.
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
I was looking at gamma-linolenic acid as a possible starting material. Ozonlysis could get you to the C6 aldehyde, isobutylmagnesium bromide to the
alcohol, and a careful elimination without shifting the double bond. Would yield both isomers, and the possiblity for the unsaturation between
carbons 5 and 6. I don't think a dehydration with phosphoric acid would give you the alkene at carbons 7-8
[Edited on 24-3-2009 by Arrhenius]
|
|
|
Nicodem
|
Threads Merged
23-3-2009 at 23:55 |
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Thanks Nicodem. I remember that thread from way back (now that I see it), but the catching point with the proposed syntheses is the dehydration. There
is no control over where the double bond goes. Also, I know (a lot better) what I'm doing now. Organic chemistry was rather foreign to me at the time.
How does this look? The starting alkene is rather easy to make compared to the target. Is the epoxide opening going to be selective enough for good
yield of the tertiary bromide? (I suspect so, but can never be sure) Any thoughts on the ? Is a simple cyanide too strong a base for SN1 with a
tertiary halide? Will it cause dehydrohalogenation instead? Does AgCN dissolve at all in methanol? with the addition of more alkali metal cyanide? The
Ag would drive the reaction by trapping the Br as it dissosciates if it does. Otherwise, I should look for a way to put an iodide or tosylate on the
end.

On second thought, perhaps that aq. HCl should be H2SO4 to try to avoid any kind of reactions with the double bond.
[Edited on 3-25-09 by UnintentionalChaos]
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|

Starting from gamma-linolenic acid. The problem lies in actually being able to recover the correct aldehyde. Whether the dehydration will work is
hard to know for sure. HIO4 or KMnO4 might be an option to cleave the alkenes, but surely you'd lose your ester protecting group. Sorry for the
alkene geometry being incorrect, I don't have chemdraw at home.
[Edited on 24-3-2009 by Arrhenius]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
OK, here is another possible route, based on stuff I did back when I had a lot of sodium. Sorry for the ugly graphics, I didn't have y usual
program along and had tp make do with a couple of freebies.
The carboxylic acid being made in 2) is 2-Propynoic acid/Acetylenecarboxylic acid/Propargylic acid/Propynoic acid and is commercially available.
1) starts by melting and dispersing the sodium in mineral oil or xylene.
2) also gives the diacid and some unreacted disodium acetylide, the reaction products derived from these should not be a problem as the diacid and its
diesters are much smaller than the target while the unreacted acetylide yields hydrocarbons.
4) may work with NaOH as well, water is cooked out of the reaction mix; or it may take NaH or NaNH2 - different acetylenes are easy or tough. It does
take time for the triple bond to work its way to the terminal position. A slight excess of base is needed, as the triple bond is pinned at the
terminal position as the salt/acetylide.
6) will require research on the proper way of reduction to give a single step of reduction to the alkene with the proper orientation.
It looks to be possible to run the first 5 steps with no intermediary isolation or workup beyond distilling excess alkyl halides out of it or
filtration to remove the inorganic salts. Good stirring is needed. Before step 6) distillation should be able to remove the diacid ester and
alkynes byproducts from 2)+3)

|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | [rquote=149800&tid=8404&
Is the epoxide opening going to be selective enough for good yield of the tertiary bromide? (I suspect so, but can never be sure)
|
Yes, in acidic media nucleophiles do attack epoxides at the carbon where the positive charge can be most efficiently stabilized (unless the epoxide
succumbs to side reactions like the various types of epoxide rearrangements, etc.). In basic media, the regioselectivity is most commonly the opposite
or mixed, because then epoxides react mostly trough a normal SN2 mechanism (yet it is nevertheless common to obtain both products, especially if the
counter ion is acidic like Li+ and similar).
| Quote: | | Is a simple cyanide too strong a base for SN1 with a tertiary halide? Will it cause dehydrohalogenation instead? |
SN1 reactions generally do not work in basic media or if they do, they are either limited to substrates where elimination can not occur, or to
nonbasic or weakly basic nucleophiles. The cyanide ion is a relatively very basic nucleophile. Besides, all this is irrelevant, since a nucleophilic
substitution on halohydrines always occurs trough the epoxide with a double inversion. It is therefore pointless to form a halohydrine from the
epoxide when you want do a nucleophilic substitution in basic media.
| Quote: | | Does AgCN dissolve at all in methanol? with the addition of more alkali metal cyanide? The Ag would drive the reaction by trapping the Br as it
dissosciates if it does. |
You can not perform a SN1 reaction in a protic nucleophilic solvent and basic media. Carbocations react with methanol and, unlike in acidic media, in
basic media this reaction is irreversible.
I suggest you to look up for Hoffman and Cope elimination reactions.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Ebao-lu
Unregistered
Posts: N/A
Registered: N/A
Member Is Offline
|
|
acid construction
Here are some other hypothetical routes.
At first one, cyclohexanone or its enamine is acylated with benzoylchloride, then the diketone is alkylated with metallylchloride. The question (?) is
the major product of basic decomposition of diketone. I suppose that cyclohexyl ring should be broken, bacause cyclohexyl's carbonyl is prone to
nucleophilic attacks, unlike benzoyl's that is stabilized by conjugation. On the other hand, the reactivity of formed deprotonated hem-diols is
contrary. The last reaction is a base-catalysed retro-ene reaction, the same like heptanal is made form castor oil. Unfortunately, the product can not
be distilled off as easy as heptanal, and i dont know if it will survive in the RM while vigourous prolonged heating(hope yes). Another option is to
use a methyl ester in the reaction and heat it with K2CO3, in a slight reduced pressure to distill the formed ester.
Second route is a Ramberg-Backlund sulfone coupling into alkene. Sorry, the picture may contain misleading information about the radical mechanism of
halogenation, i should enquire. This reaction is generally having good yields, and the trans products are formed preferably with long-chained
substituents and when strong base (like t-BuOK) and aprotic solvent used. CBr4 can be replaced with CCl4, and halogenation can be done is situ, PTC
conditions should also be feasible. As for hydrolysis of a-ketosulfones like dicarbonyl compounds, it should be also clarified from literature, maybe
the sulfinic acid would form preferably, or the reaction is too slow.
As for Nicodem's suggested hoffmann and cope elimination, the basic hydrolysis of isobutiroyl cyclohexanone can probably give the ketone for reductive
amination.

[Edited on 31-3-2009 by Ebao-lu]
[Edited on 31-3-2009 by Ebao-lu]
[Edited on 31-3-2009 by Ebao-lu]
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
That second reaction looks particularly excellent. Of course, my choice of starting material would be quite a bit different and I'm not so sure about
the radical halogenation.
I'd start by hydrolyzing poly-caprolactone, and using HBr or conc. HCl to make the terminal alcohol a halogen, giving a 6-halohexanoic acid. I'm still
not sure about the alpha-halosulfone
I like this route in that it avoids methyl iodide, not that I'd consider it unacceptable, but why use it if you don't need to.
[Edited on 3-31-09 by UnintentionalChaos]
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
| Pages:
1
2 |