ChemDunce
Harmless

Posts: 3
Registered: 2-5-2007
Member Is Offline
Mood: No Mood
|
|
Chem Final
Hey Chem geniuses!
I am a struggling high school chem student who couldn't combine water with water without blowing something up so some help would definitley be
appreciated.
My chem final has been issued already and it is as follows:
"Put 5 mL of 3 M aluminum chloride solution in each of two test tubes. To one test tube add a few drops of 3 M sodium hydroxide solution. Then add
more sodium hydroxide solution, with stirring, until the precipitate dissolves. Treat the other test tube similarly, but use 3 M ammonum hydroxide
solution to obtain precipitation and then add more ammonium hydroxide solution. Why did the precipitate dissolve in one case and not the other?"
I carried out the experiment last Friday and basically got the desired results. The NaOH dissolved while the NH4OH did not.
Could someone please explain why one dissolved and one remained rather solidified?
The chemical equation for this is a double replacement for both solutions, is it not?
*flummoxed*
[Edited on 2-5-2007 by ChemDunce]
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Ammonium hydroxide isn't.
Look at the definition of weak acid and base, and especially, compare the Ka's of aluminum hydroxide (Al(OH)3 + H2O <--> H+ + Al(OH)4-, Ka = ?)
and ammonium ion (NH4+ <--> NH3 + H+, Ka = ?).
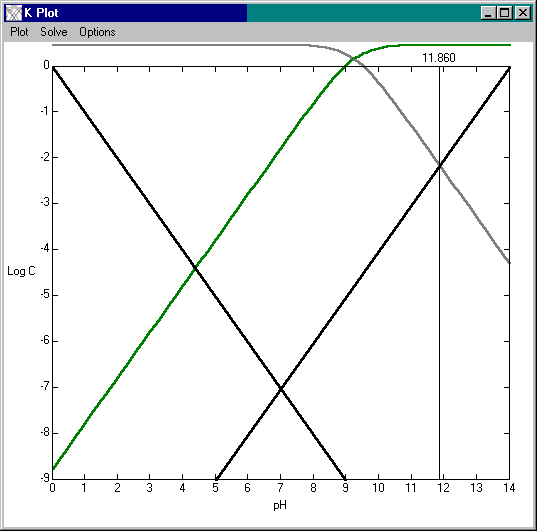
Here's a plot of the five species in solution (H+, OH-, NH4+ and NH3), give or take the activity coefficient (since this is a rather strong solution).
Most of the ammonia (99.99%) is as ammonia, not hydroxide. This gives you some idea of its basicity. Aluminum responds similarly, but aluminate is
a weaker acid so does not form a salt. Similarly, aluminum carbonate cannot exist.
Tim
|
|
|
dedalus
Hazard to Self
 
Posts: 60
Registered: 13-4-2007
Location: New York
Member Is Offline
Mood: No Mood
|
|
Nice graphic, dude!
Did you generate that with MINEQL, or whatever they're calling it these days? I played around with a DOS based version but wasn't enough of a computer
guy to take full advantage of it.
Do you know Kragten's Atlas of Ionic Equilibria?
And, I always call it "aqua ammonia", these days.
[Edited on 3-5-2007 by dedalus]
|
|
|
ChemDunce
Harmless

Posts: 3
Registered: 2-5-2007
Member Is Offline
Mood: No Mood
|
|
I appreciate the explanation, but would someone mind explaining that in layman's terms? In other words, please dumb it down a little so that I can
grasp the gist of it.
Also, which ones of these apply to the experiment?
Physical Changes
Ions in Solutions
The Elements of Group IA and their Compounds
The Elements of Group IIA and their Compounds
Oxidation-Reduction Reactions
The Elements of Group IIIA and their Compounds
Hydrolysis (acid/base) and equilibrium
The Elements of Group IVA and their Compounds
The Elements of Group VA and their Compounds
Organic Chemistry
The Halogens, of Group VIIA, and their Compounds
Rates of Reaction, a Study of Kinetics
The Chemistry of Surfaces, Colloids, and Diffusion
A study of principles
The properties of substances
Analytical Chemistry, Qualitative Analysis
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Didn't you already get this explained to you on chemicalforums? You shouldn't need that last one explained...It's a classic example of a bullshit
question just like listing the themes in some book for english class. This isn't a forum for us to do your homework.
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
dedalus
Hazard to Self
 
Posts: 60
Registered: 13-4-2007
Location: New York
Member Is Offline
Mood: No Mood
|
|
Go work for a plating shop. Out of sheer laziness, fail to analyze the zinc plating bath till the metal goes down to the point where the bath won't
plate no more.
Now, take 50 lbs of sodium hydroxide beads, and dissolve a bunch of zinc oxide in it. Get burns all over your forearms fixing your own screw up.
Now, repeat the experiment with ammonium "hydroxide".
Report your observations after your release from the hospital.
Hint, hint: what does "amphoteric" mean?
I did part 1. Because I'm such a chem genius (I got a C in that course you're taking, BTW) I was able to omit part 2.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
| Quote: | Originally posted by dedalus
Nice graphic, dude!
Did you generate that with MINEQL, or whatever they're calling it these days? |
Comes with the class. Appears the prof knows a bit of -- whatever language it's written in. I'll warn you, it's still buggy.
http://www.beloit.edu/~chem/Chem220/
KPlot a little down from the top there.
It's insane, for big problems, instead of doing calculations for some 13 species in solution (for instance, EDTA acid dissociation and ammonia
complexation simultaneously occurring on some metal center), he puts printouts on tests so we just fill in the blanks. And we do stuff like label the
curves on plots like these.
|
|
|
ChemDunce
Harmless

Posts: 3
Registered: 2-5-2007
Member Is Offline
Mood: No Mood
|
|
They don't call me Chemdunce for no reason.  Thanks for your help, guys. Thanks for your help, guys.
|
|
|
dedalus
Hazard to Self
 
Posts: 60
Registered: 13-4-2007
Location: New York
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by 12AX7
[
Comes with the class. Appears the prof knows a bit of -- whatever language it's written in. I'll warn you, it's still buggy.
http://www.beloit.edu/~chem/Chem220/
KPlot a little down from the top there.
It's insane, for big problems, instead of doing calculations for some 13 species in solution (for instance, EDTA acid dissociation and ammonia
complexation simultaneously occurring on some metal center), he puts printouts on tests so we just fill in the blanks. And we do stuff like label the
curves on plots like these. |
|
|
|
dedalus
Hazard to Self
 
Posts: 60
Registered: 13-4-2007
Location: New York
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by 12AX7
| Quote: | Originally posted by dedalus
Nice graphic, dude!
Did you generate that with MINEQL, or whatever they're calling it these days? |
Comes with the class. Appears the prof knows a bit of -- whatever language it's written in. I'll warn you, it's still buggy.
http://www.beloit.edu/~chem/Chem220/
KPlot a little down from the top there.
It's insane, for big problems, instead of doing calculations for some 13 species in solution (for instance, EDTA acid dissociation and ammonia
complexation simultaneously occurring on some metal center), he puts printouts on tests so we just fill in the blanks. And we do stuff like label the
curves on plots like these. |
One of my great chemical fascinations is hydrolysis Here's a neat thing - what color is ferric nitrate?
It's purple. That's the color of the Fe3+(H2O)6 ion. Now, add water. This complex ion is so acidic, it can't wait to kick off a proton and become
Fe3+(H2O)5 OH, which is brown.
That book I alluded to, Kragten's, is an atlas of such equilbria, along with a plethora of of graphs showing complexing agents effect on them. All
done with '70's computer technology.
[Edited on 4-5-2007 by dedalus]
|
|
|