Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
One for the Energetics Boffins
I am studying the preparation of 2-nitrophenylhydrazine with a view toward making my own HBTU (1-hydroxybenzotriazole) peptide coupling reagent.
The usual prep involves diazotizing 2-nitroaniline and then reducing the diazonium salt with sulfite (Na, K, NH4)
Some of our Euro colleagues have objected because they can't buy NaNO2.
So I am looking at reacting hydrazine sulfate or hydrate with 2-nitrochlorobenzene or 2-nitrobromobenzene in a SnAr fashion. I know this works for the
dinitrochlorobenzenes and for picramide.
I also know that 2-nitrochlorobenzene reacts almost quantitatively with excess 25% NH4OH in an autoclave at 170 C and a pressure of about 500 psig
(autogenous).
So, I was considering doing same with hydrazine hydrate say 60-65%.
My question to you is: anything wrong with this idea?
After all we are talking about a substituted nitrobenzenem and hydrazine - albeit it, diluted with water.
Certainly not anhydrous hydrazine.
First and foremost is this safe?
Secondly will it work?
And finally, why is this not a standard method?
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
It should be safe enough. Sandmeyer will go. Hydrazine SnAr should also go, but might require a promoter (although a heavily electron deficient ring
does favor both electrophilic attack and ipso additions). Give it a try.
Along similar lines, I have a new favorite chemical (at least for today):
1,1-diphenyl-picrylhydrazyl free radical. The free radical is deep purple, like KMNO4 (the radical provides resonant conjugation between the
diphenylamine group and the trinitroaniline group. Reaction of this compound with either an antioxidant (such as trolox or caffeic acid) or a small
radical (such as .OH) destroys this "conjugation" to yield only the yellow from the trinitro group. I'll look up it's preparation; it might be useful
in the context of your desired product.
Take care,
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Sounds neat!
It should be mentioned that most of the phenylhydrazines are valuable (useful and expensive) reagents.
Most of are well aware I am sure of phenylhydrazine's role in sugar analysis.
Most of us probably also recognize that 2,4-dinitrophenylhydrazine is a standard qualitative reagent for derivatizing aldehydes and ketones.
Less well known is that o-nitrophenylhydrazine is a colorimetric reagent for identification and determination of carboxylic acids as well as other
carbonyl compounds and is used in HPLC as well.
Same is true of p-nitrophenylhydrazine.
Brother O3 just elaborated a use for picry hydrazine (sym-trinitrophenylhydrazine) which is prepared (I guess) from picryl chloride.
So I think it's a good idea to know how to make all of these, seeing the prices for the commercial products and the ever increaing costs and
restrictions on transport.
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
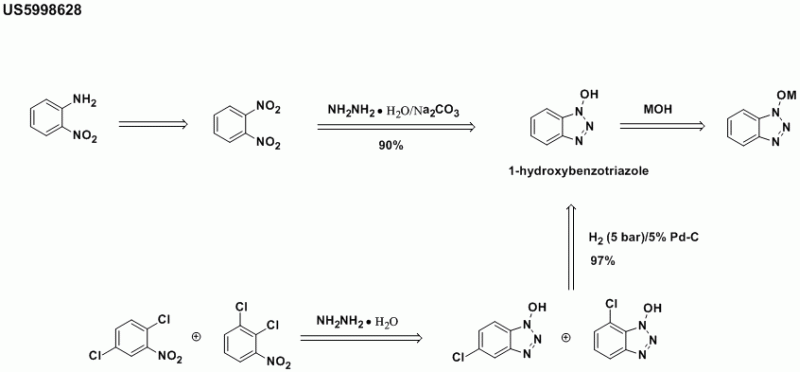
I'm surprised that there hasn't been any discussion of the explosive nature of this reagent. HOBt & this problem are discussed in this patent: http://www.pat2pdf.org/patents/pat5998628.pdf.
It states that, at least in Germany, HOBt is strictly regulated due to its explosive tendencies. They appear to get around this by converting it to
the alkali or alkaline metal salts.
I've read that the UN reclassified this compound recently & that it is no longer transportable by air as a rfesuolt.
[Edited on 9-8-2008 by Ritter]
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Probably because the "explosive" nature of HBTU has been greatly overhyped by the EU HBTU is a common and useful peptide coupling reagent.
But the EU reclassified it a few years ago and now the pharmaceutical industry has to jump throiugh hoops to ship and store HBTU in bulk.
This appears to many peptide chemists to be a ploy to force end users (the pharm industry) to switch to certain halogenated derivatives that are more
expensive by far.
That suggests a sweetheart deal between some Brussels technocrats and certain European chemical giants - a situation that is hardly unprecedented if
one knows anything at all about opaque business and bureaucratic practices in the glorious new Europe.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
The related compound HOAt is listed as having an explosion hazard. It is the 7-aza analog of HOBt.
I searched for several other N-containing analogs of HOBt but didn't come up with anything. I would think that higher N-content analogs of HOBt would
be interesting to synthesize & evaluate for their thermochemical characteristics.
[Edited on 9-8-2008 by Ritter]

Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
HBTU has been widely used in both lab abd industry as a peptide reagent for many decades. So there is a large body of experience with its handling and
storage. It has never been used as an energetic material.
That pyridine analog you just presented is much more expensive than HBTU. SO is HCTU which is subject of the patent you posted. The ring nitrogen or
the Cl-substituent stabilize the benzotriazole ring system, but at a huge cost premium that the pharm industry is loathe to accept, but which the
chemical manufacturers would love to force down their throats with the help of their pet regulators. The result will be higher consumer prices for the
peptide drugs and I do not see that as a good thing, just to fatten the purses of a few manufacturers.
[Edited on 10-8-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
| Quote: | Originally posted by Sauron
HBTU has been widely used in both lab abd industry as a peptide reagent for many decades. So there is a large body of experience with its handling and
storage. It has never been used as an energetic material. |
| Quote: | Journal of Hazardous Materials
Volume 126, Issues 1-3, 11 November 2005, Pages 1-7
Related Articles in ScienceDirect
On the explosive properties of 1H-benzotriazole and 1H-1,2,3-triazole
Tetrahedron Letters, Volume 48, Issue 7, 12 February 2007, Pages 1233-1235
Marcus Malow, Klaus D. Wehrstedt, Steffen Neuenfeld
Abstract
For 1H-benzotriazole, no explosive properties are observable, but the relative high exothermic decomposition energy of 1590 J/g should be kept in
mind. Nevertheless, an endothermic melting barrier at 100 °C ensures safe handling at lower temperatures. For 1H-1,2,3-triazole, the exothermic
decomposition energy is as high as 2600 J/g, but explosive properties are also not detectable. Therefore, both reagents are hazardous with regard to
the exothermic decomposition potential and can be handled safely with precautions.
Tetrahedron, Volume 57, Issue 12, 17 March 2001, Pages
Copyright © 2005 Elsevier B.V. All rights reserved.
Explosive properties of 1-hydroxybenzotriazoles
K.D. Wehrstedta, , , P.A. Wandreya and D. Heitkampb
Bundesanstalt für Materialforschung und -prüfung (BAM), Working Group “Explosive Substances of Chemical Industries”, Unter den Eichen 87,
D-12205 Berlin, Germany bBayer Industry Services, BIS SUA VA, D-51368 Leverkusen, Germany
Abstract
1-Hydroxybenzotriazole and its derivatives are widely used as peptide coupling reagents. However, people are often not aware that such compounds show
explosive properties when heated under defined confinement or when subjected to mechanical stimulus. 1-Hydroxybenzotriazole (HOBt) is able to
propagate a detonation when a stronger booster is used. Sometimes explosive substances are desensitized to suppress their hazardous properties, yet
depending on the amount and nature of desensitizer, the result is often not quite satisfactory.
During the last years, some 1-hydroxybenzotriazoles were tested at BAM. The results are presented in this paper.
|
See also http://www.unece.org/trans/doc/2006/ac10c3/UN-SCETDG-29-INF2....
From the MSDS:
| Quote: | Chemical Stability: Unstable. Heating above 180°C results in rapid exothermic decomposition. Heating may cause an explosion. Light
sensitive. A dangerous self-accelerating decomposition reaction and, under certain circumstances, explosion or fire can be caused by direct
contact with incompatible substances or by thermal decomposition at and above the SADT (Self-Accelerating Decomposition Temp)
|
http://www.coleparmer.com/catalog/Msds/95379.htm
[Edited on 9-8-2008 by Ritter]
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Just the sort of exagerated hazard assesments I am complaining about.
What is happening as a result is that the pharm industry and researchers are abandoning HBTU, rejecting the adoption of HCTU etc, and instead
returning to activated esters and acyl chloride coupling methods for peptide work.
Hence the impetus for development of rapid, very mild chlorinating reagents for this application, see my thread on this of a few weeks ago for
examples.
Sic gorgeamus a los subjectatus nunc.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
2,3,5,6-tetraaminopyrazine
Discussed here _
http://www.sciencemadness.org/talk/viewthread.php?tid=13174#...
http://www.sciencemadness.org/talk/viewthread.php?tid=13174#...
http://www.google.com/patents/download/3808209_DI__NITROPYRA...
By using the following reaction scheme on 2,3,5,6-tetraaminopyrazine
1H-1,2,3-Benzotriazole
http://www.orgsyn.org/orgsyn/prep.asp?prep=cv3p0106
This product can be obtained
di(1H-1,2,3)triazolo[4,5-b,e]pyrazine
![di(1H-1,2,3)triazolo[4,5-b,e]pyrazine.gif - 4kB di(1H-1,2,3)triazolo[4,5-b,e]pyrazine.gif - 4kB](http://www.sciencemadness.org/talk/files.php?pid=255724&aid=19679)
Replacing the two hydrogens with nitro groups may yield a relatively
insensitive energetic compound. Trinitromethyl or Dinitroamine instead
give complete oxygen balance.
Synthesis of Trinitromethyl- and Dinitromethyl-Substituted Azoles Using Nitrate Salts in Sulfuric Acid
http://www.sciencemadness.org/talk/viewthread.php?tid=1970&a...
.
|
|
|