woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Thionyl chloride and acetone, what happens?
I tried to make 2,2-dichloropropanol from acetone and thionyl chloride, assuming that the following would happen:
CH3C(=O)CH3 + SOCl2 --> CH3CCl2CH3 + SO2
I dripped in a few drops of thionyl chloride in acetone and then swirled the test tube. This results in fairly quick formation of a golden yellow
liquid. There is no smell of SO2, nor does the pungent smell of SOCl2 remain. The liquid obtains a strange spicey smell, after some time, the smell
becomes objectionable.
I plunged the golden yellow liquid in a lot of water. Most of the liquid dissolves (I assume this is mainly unreacted acetone) and a yellow oily blob
remains at the bottom. This oily blob really does not dissolve. When it is shaken vigorously with water, then it breaks down in a zillion small oily
droplets, which, however, quickly collect into a single yellow blob at the bottom. The shaking makes the blob a litte turbid, probably, because of
very fine droplets of water in the yellow liquid. The yellow liquid has a spicey smell, which after some time becomes disgusting.
I only smelled this liquid VERY briefly, I'm always very careful with organics, smelling them.
Who knows what nasty I have made? I severely doubt that this is CH3CCl2CH3.
|
|
|
solo
International Hazard
    
Posts: 3967
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Reference Information
Are a-Oxothioacyl Chlorides Formed in the Reaction of Methyl Ketones with Thionyl Chloride?
Gunadi Adiwidjaja, Horst Giinther, and Jiirgen Voss
Angewandte Chemie International Edition in English Volume 19, Issue 7 , Pages 563 - 564
Attachment: Are a-Oxothioacyl Chlorides Formed in the Reaction of Methyl Ketones with Thionyl Chloride?.pdf (222kB)
This file has been downloaded 2013 times
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
About the only thing that popped out of that reference was a compound I first encountered a few days ago, which is product of the reaction between
P4S10 and anisole at elevated temperature and pressure, and with a large excess of anisole.
It turns out that such high temperatures and an autoclave are not necessary. An Org.Syn. procedure reports an 82% yield from a 10:1 excess of anisole
with P4O10 at reflux in 2 hours.
The product, now known as Lawesson's reagent, is a convenient thiating reagent, deemed more reproducible than P4O10 from which is is made.
[Edited on 2-10-2007 by Sauron]

Sic gorgeamus a los subjectatus nunc.
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
| Quote: | Originally posted by solo
Reference Information
Are a-Oxothioacyl Chlorides Formed in the Reaction of Methyl Ketones with Thionyl Chloride?
Gunadi Adiwidjaja, Horst Giinther, and Jiirgen Voss
Angewandte Chemie International Edition in English Volume 19, Issue 7 , Pages 563 - 564
|
I cannot really determine from this PDF-file, what is formed now. It only mentions a very complex mix, obtained from reaction of SOCl2 and CH3COCH3.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
I suspect you o have a complex mixture. Note that in the PDF the reaction of acetone and SOCl2 takes place in the presence of pyridine. If nothing
else this sucks up the HCl produced, which otherwise can cause condensation reactions in the acetone (see making mono-halo-acetones where some base is
used to capture the HX)
I'd expect their #4 trisulfane to be reasonably stable, but under the influence of the HCl could react to squeeze out at least one sulfur, which would
result in a sulfide or disulfide along with elemental sulfur.
I wouldn't expect SOCl2 to convert the carbonyl oxygen to the gem-dichloride, do you have a reference for that?
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I do not have a reference for that reaction, it was just personal thinking about what could happen. SOCl2 can react with oxides to make chlorides,
itself being converted to SO2, but this is mostly inorganic stuff. I realize that with ketones things are very different.
I could use triethylamine for taking away any HCl, formed in the reaction, but doesn't that react with thionyl chloride itself? I read something about
nitrogen mustard-gases being formed with thionyl chloride and ethanol-amines. I don't have ethanol amines, but even if my triethyl amine only contains
0.1% or so of (tri)ethanol amine, then I could make some nitrogen mustard, and that is the last thing I want in my home lab. Maybe I am a little bit
too concerned, but better safe than sorrow.
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Triethylamine is a good substitute for pyridine, and it won't react with SOCl2.
And no, triethylamine doesnt contain any ethanolamines. I cant imagine any side reaction in the manufacture process (ethanol + ammonia over catalyst)
that could give rise to that kind of impurity.
Note that triethylamine hydrochloride will separate as a fine crystalline precicpitate in reactions that produce HCl. And you will still not get any
definite compound from the acetone.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
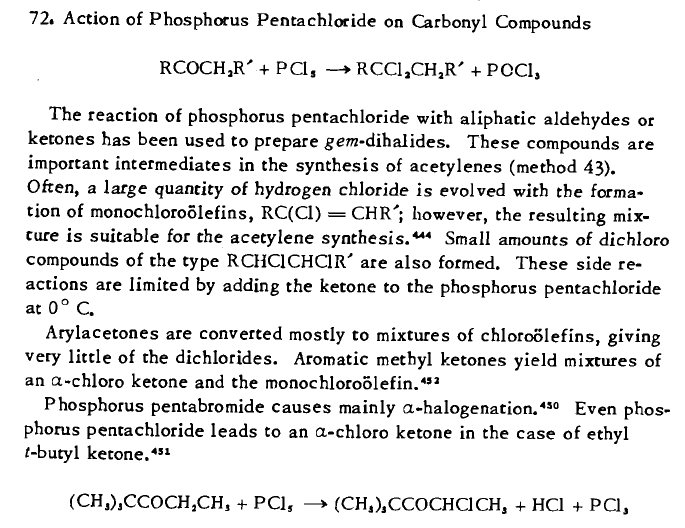
PCl5 is the only reagent of which I'm know that is able to form gem-dichlorides from ketones.
From Synthetic Organic Chemistry (Wagner&Zook, 1953), page 105:
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
So PCl5 does this sometimes, maybe.
There are some caveats. Aryl ketones give a-chloroolefins. Not gem-dichlorides.
And PCl5 is not only not OTC it is often quite restricted, certainly in USA per DHS list, certainly in my country, certainly by CWC for whatever that
is worth. Can members in the EU buy it? Probably not.
Those who can buy red P can of course make their own, bring loads of Cl2.
Not an option for me.
[Edited on 3-10-2007 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
I just got a new GC-MS in at work today. I think that I shall put a "couple of drops" of thionyl chloride into some acetone, incubate a bit, then
dilute with DCM and see what we get.
I will do some preliminary tests with a large excess of acetone (and some tests with water washes) to try and avoid putting unreacted SOCl2 on my
column . .
Oh yes, IIRC SOCl2 and pyridine is a powerful dehydrating reagent that is referred to in Fieser vol. 1. I have found, in the past, that a trace of
SOCl2 left in your acyl chloride frequently leads to instant "tar" formation when used with pyridine and your alcohol or amine. Boo!
I'll let you know how this goes,
O3
[Edited on 4-10-2007 by Ozone]
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
"I just got a new GC-MS in at work today. I think that I shall put a "couple of drops" of thionyl chloride into some acetone, incubate a bit, then
dilute with DCM and see what we get."
I'm intensly jealous! I just fired up my humble IR and it seems to have survived the travel and downtime. It was pretty dicey coming from a desert
climate out to the west coast. The want to give me an older one that has big prisms in it. I shall have to move it in a heated van.
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Thanks! (7890GC + 5975C MSD). I'm glad to hear that your DIR is working! (esp. w/roamingnome's shipping catastrophe).
To acetone (1mL, ACS) was added SOCl2, 5uL. The sample was "incubated" at ambient T, 22°C for 6 hr. I ran it:
45 C 2 min
300 C, 15 C/min, hold 10min
on a DB-5 (30m x 0.25mm x 0.25um film).
I was unable to detect anything other than acetone-dimer (and bis(2-ethylhexyl phthalate ). ).
The check out got a S/N = 190 on 1pg octafluoronapthalene this morning. Could this possible not be sensitive enough ? Tune was good. ? Tune was good.
Oh, and 'Rox, it seems that your experiments might go a bit more smoothly if you had a self-grooming cat  . (I could not justify opening another post just for that in the Ether thread). . (I could not justify opening another post just for that in the Ether thread).
Cheers,
O3
[Edited on 4-10-2007 by Ozone]
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Could it be that you did not add enough SOCl2? I understand that you have to be very careful and don't want SOCl2 in the GC-MS. It indeed surprises me
that you don't find anything interesting. I would at least expect some chlorine-containing material to be detected.
Anyway, I appreciate very much that you did such a test  ! !
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
No problem!
Or, the interesting stuff reacted with my injection liner/seal. Or, it came out within my solvent delay. I'll try it again, maybe next week, with a
larger amount of SOCl2. I will have to add DCM or toluene and quench it with water (brine) before running it. Hopefully, the products will exchange
into the organic phase and we will be able to see something.
What grade of acetone did you use?
Cheers,
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
The chemicals I used are general purpose laboratory reagents, nothing fancy, but certainly better than hardware store stuff.
The thionyl chloride is 99+ % grade, according to the label. It has a faint yellow/brown color.
The acetone is so-called GPR-grade. It is perfectly colorless.
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
GPR (general purpose reagent, IIRC) is OK. My thionyl chloride is colorless (but I store it under N2 in the freezer).
Hmm. Just checking to make sure we weren't looking at "homebrew" acetone, heck even tech (drum) grade has ~0.5% "stuff" when evaporated.
Cool, we'll see,
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
Sergei_Eisenstein
Hazard to Others
  
Posts: 290
Registered: 13-12-2004
Location: Waziristan
Member Is Offline
Mood: training
|
|
| Quote: | | Or, the interesting stuff reacted with my injection liner/seal. Or, it came out within my solvent delay. I'll try it again, maybe next week, with a
larger amount of SOCl2. I will have to add DCM or toluene and quench it with water (brine) before running it. Hopefully, the products will exchange
into the organic phase and we will be able to see something. |
Your liner should be deactivated. The split/splitless seal usually is gold plated. If a reaction occurs, the capillary will the target of choice. Poor
thing... 
Woelen's target compound (2,2-dichloropropane) has bp 70-71. I don't know your solvent delay time (3 to 4 min?), but I think that the target compound
elutes very close to acetone, and considering that the amount of acetone is much higher than the amount of 2,2-dichloropropane, I also suspect that
the signal of the latter is efficiently "masked" by acetone. Also, you may want to consider sampling the headspace instead of injecting a liquid
sample. Unless the products formed during the acetone/SOCl2-reaction are not volatile enough, this analysis method may be prefered.
damnant quod non intelligunt
|
|
|