| Pages:
1
2 |
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
Magpie, I always enjoy your experimentals.
I don't want to reduce anything. I don't even expect worthwhile yields from that, but it utilizes some interesting chemical reactions.
| Quote: | if you esterify the COOH and treat that with DIBAL,you could reduce it to the aldehyde.then to get alcohol,you could treat it with dithionite and to
get hydrocarbon,you could do clemmenson.I think DIBAL can be made at home,as isobutanol is available
http://en.wikipedia.org/wiki/Diisobutylaluminium_hydride#Pro...
|
I know about DIBAL. It's a commonly taught in sophomore organic chemistry. I'd love to see an experimental of you synthesizing it at home.
| Quote: | I couldn't follow your reaction as you haven't mentioned your starting product,but if you are starting off with an ester of acid lower than adipic
acid,there might be a problem
|
The ring expansion gives it away in a retrosynthetic analysis.
|
|
|
Crowfjord
Hazard to Others
  
Posts: 390
Registered: 20-1-2013
Location: Pacific Northwest
Member Is Offline
Mood: Ever so slowly crystallizing...
|
|
There are ways to reduce carboxylic acids or their esters to the corresponding alcohols using modified sodium borohydride systems. These methods are
probably safer than LAH or its modifications. See attached for some examples.
Attachment: enhancing.nabh4.reactivity.and.selectivity.pdf (315kB)
This file has been downloaded 595 times
Attachment: Reduction of Carboxylic Acids with Sodium Borohydride and an Electrophile.pdf (104kB)
This file has been downloaded 451 times
Attachment: Chemoselective Reduction of Esters by Sodium Borohydride.pdf (3.2MB)
This file has been downloaded 2692 times
|
|
|
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
Probably also worth noting, to anyone interested but not yet familiar, that respective acyl halides are easily reduced to aldehydes and alcohols.
Yields in my example DOI were excellent. Sometimes this allows for different solvent systems, such as DCM/water, which might be more available than
THF for some.
However, this presumes the use of home agents such as thionyl chloride or alternatives discussed elsewhere in the forum with accompanying
experimentals. Not sure how available NaBH4 is everywhere, but there are a few potential OTC avenues I'm aware of.
Example in DOI:10.1080/00397910903340645
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
Hey Crowfjord-nice set of refs-thank you!
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I don't know of any OTC source of NaBH4, which is a little surprising as it is used by the ton for paper bleaching. Fortunately we can buy it from
Elemental Scientific. I have not used it since organic class at college where we made isoborneol from camphor (a ketone).
There's another way to make alcohols from esters: the classic Bouveault-Blanc synthesis, which uses elemental Na. It's somewhat tedious but it does
work:
http://www.sciencemadness.org/talk/viewthread.php?tid=18625#...
[Edited on 23-2-2015 by Magpie]
[Edited on 24-2-2015 by Magpie]
[Edited on 24-2-2015 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
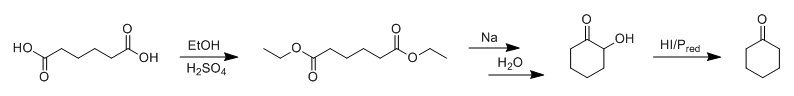
Since we're on the topic of over-the-counter chemistry, adipic acid, novel cyclohexanone syntheses and ester reductions using elemental Na, what about
something like this: Adipic acid is first converted to a diester, which then undergoes intramolecular cyclisation on reduction by Na to give a cyclic
α-hydroxyketone via acyloin condensation. The 2-hydroxycyclohexanone is then reduced to cyclohexanone using hydroiodic acid and red phosphorous.
(should work due to the adjacent pi-system of the carbonyl group)
Obviously this wouldn't be a very practical way to make it, however, as elemental sodium, red phosphorous and iodine are way more valuable than
cyclohexanone, and are a hell of a lot harder for most people to obtain anyway. I guess you could try to recover your iodine during the work-up,
though.
Maybe I'll try it on a small scale one of these days just for the hell of it. 
|
|
|
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
I like that.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Chemosynthesis  |
I know about DIBAL. It's a commonly taught in sophomore organic chemistry. I'd love to see an experimental of you synthesizing it at home.
|
I might have mis understood,are you mocking me ?
| Quote: | | The ring expansion gives it away in a retrosynthetic analysis. |
yes,I know the ring expansion,but what I was trying to say was that if lower acid than adipic acid is used,then cyclohexadione will form instead of
the expected product,because a six carbon ring is more stable
IIRC,NaBH4 is a watched chemical
I had this crazy idea,suppose you did a birch reduction of acetanilide.you would normally get a 1,4 dihydro product,but if you carried out the
reduction at high temp,the 1,4 will isomerise to 1,2 and the reduction will take place again to give (hopefully) N-(1-cyclohexene) acetamide,which can
be hydrolysed to get cyclohexanone
or you could start off from nitrobenzene
http://en.wikipedia.org/wiki/Birch_reduction#Second_step_of_...
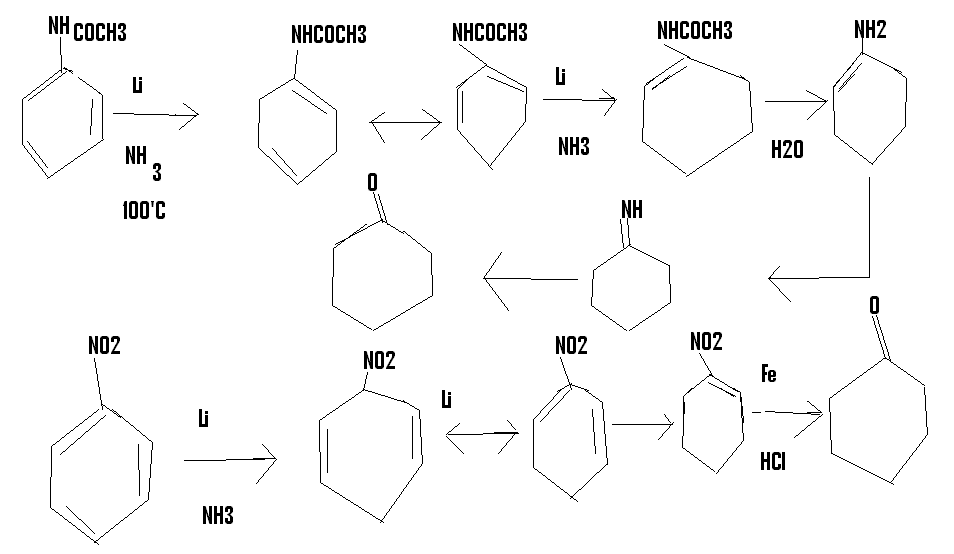
[Edited on 24-2-2015 by CuReUS]
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Darkstar  | Since we're on the topic of over-the-counter chemistry, adipic acid, novel cyclohexanone syntheses and ester reductions using elemental Na, what about
something like this: Adipic acid is first converted to a diester, which then undergoes intramolecular cyclisation on reduction by Na to give a cyclic
α-hydroxyketone via acyloin condensation. The 2-hydroxycyclohexanone is then reduced to cyclohexanone using hydroiodic acid and red phosphorous.
(should work due to the adjacent pi-system of the carbonyl group)
Obviously this wouldn't be a very practical way to make it, however, as elemental sodium, red phosphorous and iodine are way more valuable than
cyclohexanone, and are a hell of a lot harder for most people to obtain anyway. I guess you could try to recover your iodine during the work-up,
though.
Maybe I'll try it on a small scale one of these days just for the hell of it. 
|
Treatment of the adipate ester with sodium in ethanol will yield the b-ketoester; ethyl 2-oxo-cyclopentanoate (Dieckmann condensation). Hydrolysis and
decarboxylation would afford cyclopentanone. If you want the acyloin product, you'd need to use 4 eq. TMS-chloride avoid the
Dieckmann.
[Edited on 24-2-2015 by DJF90]
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
@ CuReUS:
If you're going to go that route, why not just do a Benkeser on phenol? Should give well over 90% cyclohexanone if you don't screw up the hydrolysis
at the end.
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
Quote: Originally posted by DJF90  |
Treatment of the adipate ester with sodium in ethanol will yield the b-ketoester; ethyl 2-oxo-cyclopentanoate (Dieckmann condensation). Hydrolysis and
decarboxylation would afford cyclopentanone. If you want the acyloin product, you'd need to use 4 eq. TMS-chloride avoid the
Dieckmann. |
The reduction isn't done in ethanol, it's done in an inert, aprotic solvent. The acyloin will be the primary product.
|
|
|
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
Not mocking. Just not sure how on earth you'd make DIBAL or really any metal hydride at home.
| Quote: |
yes,I know the ring expansion,but what I was trying to say was that if lower acid than adipic acid is used,then cyclohexadione will form instead of
the expected product,because a six carbon ring is more stable |
Not a problem, even though you're not being specific. Take succinic acid, for example. Proceed through Dieckmann cyclization and decarboxylation to
the dione, protect with one equivalent ketal, and then (the part I haven't done to this substrate) reduce via Wolf-Kishner or similar.
| Quote: | | IIRC,NaBH4 is a watched chemical |
Watched doesn't mean not sold.
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Darkstar  | Quote: Originally posted by DJF90  |
Treatment of the adipate ester with sodium in ethanol will yield the b-ketoester; ethyl 2-oxo-cyclopentanoate (Dieckmann condensation). Hydrolysis and
decarboxylation would afford cyclopentanone. If you want the acyloin product, you'd need to use 4 eq. TMS-chloride avoid the
Dieckmann. |
The reduction isn't done in ethanol, it's done in an inert, aprotic solvent. The acyloin will be the primary product. |
Sorry, the mention of ethanol was meant for the dieckmann route. Even in xylenes (typical solvent) you need the TMS-chloride to favour the acyloin, as
the byproduct ethoxide will otherwise lead to the dieckmann product.
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
Quote: Originally posted by DJF90  |
Sorry, the mention of ethanol was meant for the dieckmann route. Even in xylenes (typical solvent) you need the TMS-chloride to favour the acyloin, as
the byproduct ethoxide will otherwise lead to the dieckmann product. |
I'm not arguing that using TMSCl won't improve overall yields, because it certainly will. It's just that the whole point was to come up with a novel
synthesis that uses OTC reagents.
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Darkstar  | Quote: Originally posted by DJF90  |
Sorry, the mention of ethanol was meant for the dieckmann route. Even in xylenes (typical solvent) you need the TMS-chloride to favour the acyloin, as
the byproduct ethoxide will otherwise lead to the dieckmann product. |
I'm not arguing that using TMSCl won't improve overall yields, because it certainly will. It's just that the whole point was to come up with a novel
synthesis that uses OTC reagents.
|
Of course, and I'm glad that we agree. But, I have a feeling that you're going,to struggle getting even 1:1 acyloin:dieckmann in the absence of a
trapping reagent.even if you're wiling to take the low yield, you're going to have to separate that mixture somehow. First guess would be
distillation, but I'm not sure without checking literature values that they'd be easily separable.
[Edited on 24-2-2015 by DJF90]
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Darkstar  | @ CuReUS:
If you're going to go that route, why not just do a Benkeser on phenol? Should give well over 90% cyclohexanone if you don't screw up the hydrolysis
at the end.
|
the literature says that birch reduction cannot be done on phenols.I don't know why.and benkeser is a modified birch reduction.the phenol would have
to be esterified or etherified first.
also phenol is a very bad chemical to work with.Its hard to get out of the bottle,freezes up in the test tube and also burns you if you aren't
careful.It also loves to get oxidised and polymerises rapidly to give tarry crap.last but not the least,its not OTC.
Also,doing an acyloin condensation is no joke because the reaction must be done in an N2 environment as traces of O2 reduces the
yield
[Edited on 3-3-2015 by CuReUS]
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
I have a reference claiming that cyclohexanone was obtained via a Benkeser on phenol at 96% yield. I'll try to dig it up.
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
Reference
Page 16 of .pdf, bottom paragraph, left side:
"Furthermore, phenol and β-phenylethyl alcohol have been reduced under Benkeser conditions. Thus, phenol is converted to cyclohexanone in 96% yield
by lithium in methyl- or ethylamine provided the hydrolysis of the reaction mixture is carried out rapidly with little lithium remaining."
Don't be so quick to assume! 
|
|
|
| Pages:
1
2 |