| Pages:
1
..
22
23
24
25
26
27 |
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Equilibrium moisture content finally appears to have been reached. Here are a couple of pictures of the dry crude yield. From 150g of erythritol, 240g
of crude ETN was obtained.
The crude product was recrystallized, but it will need time to dry. The 240g of crude product was added by the spoonful to about 720mL of methanol
which was previously brought almost to the boil. With the exception of a sub gram quantity of solid material all material dissolved within seconds. A
hot filtration was performed, but because of the simple filtration apparatus and a -15C ambient temperature rapid cooling caused the filter to clog
when the process was only about 2/3 complete. Nearly all material was recovered in spite of this, but it was inconvenient to say the least. There is a
big difference between processing 24g of ETN and 240g! I need to develop more finesse in the purification part of the process that much is evident. In
this particular case a hot filtration hardly seemed worth it given the tiny amount of undissolved material, especially if not properly equipped to do
one. The undissolved solid material maybe could have been removed some other way.
 
[Edited on 7-3-2015 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Issues Associated with ETN Recrystallizations from Methanol
Performing the ETN synthesis described in the last few posts has caused me to remember a few things about problems associated with ETN
recrystallizations from hot methanol and to search for explanations. The following is a U2U exchange with Philou where he gives a good explanation of
what is likely happening at the higher recrystallization temperatures.
Quote:
Hello Philou,
I have noticed when recrystallizing ETN from methanol that the solution will turn a yellow-orange color and also that a lot of yield weight will be
lost at the same time. This only seems to happen at temperatures approaching the melting point of ETN. For instance, if the methanol is only heated to
50C the solution seems to stay mostly clear and the final yield is greater. The yellow-orange material produced can be fairly easily washed from the
precipitated ETN, but when not well washed it was noted that as the ETN was drying there was an acrid, irritating to the eyes and nose smell which
came from the sample on close inspection.
I believe the ETN or lower nitrates are decomposing. Rosco suggested maybe transesterification when I mentioned it to him. Do you have an idea about
what is happening?
Thanks,
Hennig
Hi HB,
Feel free to post this into your tread!
Yes Rosco is right transesterification seems plausible reason.
Usually it has to be catalysed by a base or an acid but owing to the "high heat" involved in your recrystalization process there are two concomittant
process at work:
1°) Due to the heat, the equilibrium is set faster and some CH3-ONO2 is formed while trinitroerythritol (hydroxy in position one or two?) is formed;
usually nitration or halogenation occurs faster at the external CH2OH what would tend to mean that the internal CHOH are more hydrolysable (or
methanolisable) and would exchange faster their nitrate, so following me it is OH in position 2. Then the hydroxytrinitroerythritol is more water of
methanol soluble and would disfavour correct crystallization of your ETN.
2°) The traces of CH3-ONO2 are hydrolysed or oxydised by the too high a heat yielding HNO3 and NxOy with CH2=O (formol-acrid smell), HCO2H (formic
acid) and CO2 + H2O. Then acidic HNO3 and HCO2H will help further catalysis for transesterification. The NxOy will then oxydise some of the
hydroxytrinitroerythritol into a ketonic variant more prompt to hydrolysis of the viccinal nitrate ester groups and thus accelarating the decay. End
Quote
In the past if the recrystallization was done at the lower temperature, typical yields were normally about 1.4g of recrystallized product per gram of
erythritol, whereas yields could go as low as 1g of recrystallized product per gram of erythritol if the recrystallization was done close to, or at,
the boiling point of methanol. I would need to do more tests, but I remember from half a dozen or more smaller scale syntheses, done in the last few
years, that even lowering the methanol solvent temperature to 50C would prevent all or nearly all of the yellow-orange color and yield loss. Lower
solvent temperatures will mean that a certain amount more solvent will need to be used however.
The following picture shows the solution of 240g of crude ETN in methanol which had been carelessly let boil for 5-10 minutes.

[Edited on 13-3-2015 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
ETN: Recrystallized Yield & Melting Point Determination
Equilibrium moisture content was again reached and the recrystallized yield is better than I predicted. From the 150g of erythritol started with, 205g
of recrystallized ETN with a melting point of ca. 60-61C was obtained, or about 1.37g of high purity ETN per gram of erythritol started with.
The plate on the left, in the attached image, holds the ETN which precipitated on cooling and plugged the filter when a hot filtration was attempted
(dry weight 82g). The plate on the right holds the ETN which precipitated from the solution which passed the filter, some of which was precipitated by
dilution of the methanol with water (dry weight 123g). I suspected that the purity would be low, but surprisingly both samples have basically the same
melting point somewhere between 60 and 61C.
A little greater care could have been used to wash the yellow-orange impurity from the recrystallized product.
Despite the fairly decent results this time around, I have definitely noticed in the past that low yields and acrid smelling yellow-orange material
production were associated with high solvent temperatures during recrystallization.
   
[Edited on 14-3-2015 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
markx
National Hazard
   
Posts: 646
Registered: 7-8-2003
Location: Northern kingdom
Member Is Offline
Mood: Very Jolly
|
|
For the sake of promoting safe(er) practices I have to note that heating a glass container full of highly flammable and highly explosive liquid
directly on a hotplate is not the best of the available options. A preheated waterbath with no active sources of heat around would suit much better
for this situation. As we know there has already been a tragic event that happened under the very same conditions and with the same reagents involved.
I trust it is our duty to learn from that and take the necessary precautions to prevent the reoccurence of similar accidents to the best of our
abilities.... please be safe, fellow experimenters!
[Edited on 16-3-2015 by markx]
Exact science is a figment of imagination.......
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Very true, a preheated water bath would be much safer. Using a glass vessel directly on a hotplate is a little more convenient, but it really isn't
worth the risk.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Nitroglycerin - Fast and Effective Residual Acidity Neutralization Using Overhead Stirring
I have not used the overhead stirrer to make NG yet, but I was just thinking how well it could work for speeding release and neutralization of
residual acidy. If no form of agitation is used nitroglycerin will sit as a blob on the bottom of the container of bicarbonate solution and acid
neutralization is extremely slow. The overhead stirrer should be an effective and safe way to make the NG blob roll around, exposing fresh surfaces
and greatly accelerating acid release and neutralization. The blade of the overhead stirrer can be positioned well away from the bottom and sides of
the vessel and its speed can be easily adjusted to that which is most effective and safe.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
In the natural diffusion of the trace acid through the NG to find the inteface with the water basic, there is a gradient of concentration of acid in
the NG ("high" in the core of the NG and low close to the interface) and of the concentration of base in the water (high in the core of the solution
and low close to the interface).
Forced agitation will speed up the encounter of the acid traces and the base and the profile of concentration will be more constant at all times
through the entire NG and the entire water. The interface will be subject to a higher concentration variation without gradient on a short distance.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
What I was thinking though I didn't state it above was that the overhead stirrer blade could be set high, well above the NG sitting at the bottom of
the container of sodium bicarbonate solution. The drag forces could be set to give the right amount of NG agitation by controlling the speed of mixing
of the water above since drag force depends on velocity. I haven't tried it yet, but it seems to me that it would be gentle and effective.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Tabun
Harmless

Posts: 38
Registered: 17-4-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Hennig Brand  | | Very true, a preheated water bath would be much safer. Using a glass vessel directly on a hotplate is a little more convenient, but it really isn't
worth the risk. |
Maybe it's a stupid question but what's the difference between a metal plate with x temperature and a bath with the same temperature?I mean...you can
control the temperature with these things and I don't think you're going to turn it to...let's say 100 degrees Celsius to heat something to 50 degrees
Celsius.
It's not the same with open flame but with an electric hotplate(or however it's called)...
|
|
|
Molecular Manipulations
Hazard to Others
  
Posts: 447
Registered: 17-12-2014
Location: The Garden of Eden
Member Is Offline
Mood: High on forbidden fruit
|
|
Quote: Originally posted by Tabun  |
Maybe it's a stupid question but what's the difference between a metal plate with x temperature and a bath with the same temperature?I mean...you can
control the temperature with these things and I don't think you're going to turn it to...let's say 100 degrees Celsius to heat something to 50 degrees
Celsius.
|
Water and metals have very different heat capacities.
The heat capacity is the amount of heat required to raise the temperature of an object or substance one degree. The temperature change is the
difference between the final temperature ( Tf ) and the initial temperature ( Ti ).
Water's heat capacity is much higher than most metals, 4.179 Joules is required to bring the temperature of one gram of 0°C water up to 1°C. Iron's
heat cap. is 0.450 J/g at 0°C. Thus water will heat up and cool down much slower than a hotplate of similar mass. There may be other reasons as well,
but I'm a chemist, not a energetics/explosives synthesizer (not that you can't be both).
-The manipulator
We are all here on earth to help others; what on earth the others are here for I don't know. -W. H. Auden
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Water provides a very even heat without high temperature "hot spots". Unless pressurized water has a maximum temperature of 100C which is a great
safety feature. Water also tends to extinguish combustion even when hot. Hotplates generally have a fairly small heat sink, poor temperature control
and not terribly great heat transfer ability, (only contacting the bottom of the flask for starters), requiring that the plate be run at a higher
temperature for effective heating. Hotplate temperatures normally fluctuate up and down, sometimes very wildly and often much above the set point
temperature (if the set point can even accurately be set). The water bath can be a large heat sink and thermal mass which means it resists sudden
changes in temperature well. Even if left on a hotplate a reasonably sized water bath can absorb the temperature fluctuations (energy) from the
hotplate and maintain a relatively constant temperature. For extra safety a very large water bath could be preheated and used to perform the HE
recrystallization since its temperature can only drop once removed from the hotplate and there is much less chance of explosive coming into contact
with a hot surface which could result in an accident (if moved well away). An insulated vessel could prevent the water bath from transferring most of
its energy to the surroundings. High temperature surfaces are dangerous especially with ETN since it has such a low decomposition temperature.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
KesterDraconis
Hazard to Self
 
Posts: 78
Registered: 27-3-2015
Member Is Offline
Mood: No Mood
|
|
So I recently made nitroglycerin, as the next explosive on a long list to make (I hope to make the next TNT!). I did the hammer test on a brick with
about half a milliliter (I made one and a half), and I learned to respect the substance even more when after my third strike (the first two broke the
brick into three pieces and didn't detonate it, my third strike was with all my force) that little drop absorbed in a paper towel powdered a good bit
of the brick piece underneath it and knocked out my hearing in one ear for a second (I wasn't wearing hearing protection, I thought it would sound
like a loud firecracker...). Needless to say, I nearly pissed my pants.
While I am amazed with its power, and have gained a new respect for it, I also want to make more of it. Yes I know contradiction right? I primarily
want it though for the purpose of making dynamite. I am wondering, what material could I use where nitrocellulose or blackpowder deflagaration
explosion would be sufficient to detonate it , or would something like that only work on pure nitroglycerin?
(trying to stay away from more unstable primaries for the moment, though I will make them in the future, since I nearly have enough money to buy the
equipment I want)
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Good thing the whole half milliliter didn't detonate. Even a drop or two detonating is very impressive. The nice thing about dynamites is that in
general they require very small quantites of primary explosives to initiate properly. That, and they are cheap, powerful and easy to make. The better
primaries are reasonably safe if proper handling procedures are understood and followed.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
caterpillar
Hazard to Others
  
Posts: 472
Registered: 8-1-2012
Member Is Offline
Mood: No Mood
|
|
When I was young, in my city an incident with a jealousy man took place. his bride preferred another one. The rejected man visited marriage with a
bottle full of NG. Sewing needles flew across the street- one friend of mine found couple of such needles at his window-sill.
Women are more perilous sometimes, than any hi explosive.
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
Quote: Originally posted by KesterDraconis  | So I recently made nitroglycerin, as the next explosive on a long list to make (I hope to make the next TNT!). I did the hammer test on a brick with
about half a milliliter (I made one and a half), and I learned to respect the substance even more when after my third strike (the first two broke the
brick into three pieces and didn't detonate it, my third strike was with all my force) that little drop absorbed in a paper towel powdered a good bit
of the brick piece underneath it and knocked out my hearing in one ear for a second (I wasn't wearing hearing protection, I thought it would sound
like a loud firecracker...). Needless to say, I nearly pissed my pants.
While I am amazed with its power, and have gained a new respect for it, I also want to make more of it. Yes I know contradiction right? I primarily
want it though for the purpose of making dynamite. I am wondering, what material could I use where nitrocellulose or blackpowder deflagaration
explosion would be sufficient to detonate it , or would something like that only work on pure nitroglycerin?
(trying to stay away from more unstable primaries for the moment, though I will make them in the future, since I nearly have enough money to buy the
equipment I want) |
NG is easier to made than TNT. TNT is very complex.
NG is much more sensitive and a lot of hazards surround it.
I stopped playing with such things. I only use AN if I need to do something.
DON'T PLAY WITH YOUR LIFE COUNTER 
|
|
|
Tabun
Harmless

Posts: 38
Registered: 17-4-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by ecos  | Quote: Originally posted by KesterDraconis  | So I recently made nitroglycerin, as the next explosive on a long list to make (I hope to make the next TNT!). I did the hammer test on a brick with
about half a milliliter (I made one and a half), and I learned to respect the substance even more when after my third strike (the first two broke the
brick into three pieces and didn't detonate it, my third strike was with all my force) that little drop absorbed in a paper towel powdered a good bit
of the brick piece underneath it and knocked out my hearing in one ear for a second (I wasn't wearing hearing protection, I thought it would sound
like a loud firecracker...). Needless to say, I nearly pissed my pants.
While I am amazed with its power, and have gained a new respect for it, I also want to make more of it. Yes I know contradiction right? I primarily
want it though for the purpose of making dynamite. I am wondering, what material could I use where nitrocellulose or blackpowder deflagaration
explosion would be sufficient to detonate it , or would something like that only work on pure nitroglycerin?
(trying to stay away from more unstable primaries for the moment, though I will make them in the future, since I nearly have enough money to buy the
equipment I want) |
NG is easier to made than TNT. TNT is very complex.
NG is much more sensitive and a lot of hazards surround it.
I stopped playing with such things. I only use AN if I need to do something.
DON'T PLAY WITH YOUR LIFE COUNTER  |
Well...TNG is for sure safer than unstable primaries.Use the gun powder and a lead ball to shoot at the nitroglycerin.The shock should be powerful
enough to set it off.I don't know exactly why but I think hiting nitroglycerin does a better job of detonating it than igniting it.
[Edited on 11-5-2015 by Tabun]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
The better primaries are quite storage stable and have sensitivities low enough that they can be safely handled with proper technique. When you say
gun powder and lead ball I assume you mean black powder and lead ball, if so it could probably work but might not be reliable. NG will gladly detonate
at a lower detonation velocity if not initiated properly. I have never done it, but from what I have read even a .22LR can initiate dynamite at close
range, but reliability is not great. Apparently anything much bigger and faster than a .22LR is quite reliable for initiating dynamite. The speed of
the bullet has to be above the speed of sound to create a shockwave. However, even a lead ball traveling at 800fps could probably initiate pure NG,
but I am not sure what the detonation velocity would be or if it would be consistent and reliable shot to shot.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Tabun
Harmless

Posts: 38
Registered: 17-4-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Hennig Brand  | | The better primaries are quite storage stable and have sensitivities low enough that they can be safely handled with proper technique. When you say
gun powder and lead ball I assume you mean black powder and lead ball, if so it could probably work but might not be reliable. NG will gladly detonate
at a lower detonation velocity if not initiated properly. I have never done it, but from what I have read even a .22LR can initiate dynamite at close
range, but reliability is not great. Apparently anything much bigger and faster than a .22LR is quite reliable for initiating dynamite. The speed of
the bullet has to be above the speed of sound to create a shockwave. However, even a lead ball traveling at 800fps could probably initiate pure NG,
but I am not sure what the detonation velocity would be or if it would be consistent and reliable shot to shot. |
Yes,I knot TNG requires a detonator to...well,detonate properly but the idea wasn't to detonate TNG but to use it instead of a primer.If you ignite a
small quantity it deflagrates,if you hit it it detonates.That makes me think a mechanical shock will do better than heat so if you hit it hard enough
it should be at least as powerful as a primary like mercury fulminate.
BTW...by "shooting with BP and lead ball" I mean literally making a detonator good enough for the job.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Since if improperly initiated NG can have very low stable detonation velocities, and it is brisance which counts the most with initiators, it can be a
very poor initiator even compared to mercury fulminate. Also, NG tends not to perform as well in the tiny charge sizes and diameters commonly employed
with initiators.
The following was taken from "Military Explosives":
Mercury fulminate detonation velocity at 4.17g/cc is 5400m/s.
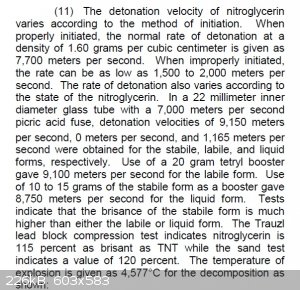
[Edited on 12-5-2015 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
I really don't like to play with NG. I synthesized it twice and had a hard headache but i might do it again in future for discovering shaped charges.
Anway, I didnt see any NG mixture with Al powder  . .
I think Al if added to AN or other mixtures it increases the power of detonation a lot.
any reason why we don't use Al powder added to NG , PETN ,... ?
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
| Quote: | | I synthesized it twice and had a hard headache |
A cup of strong coffee usually takes care of that-
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Adding aluminum powder, up to a certain percentage, does normally increase the explosive power, but it also normally lowers the brisance.
Aluminum power is a relatively expensive fuel, so it is normally not used for general blasting. It definitely can be added to dynamites and ammonium
nitrate explosives, etc, and will often significantly increase energy and power. Aluminum is even sometimes added to very oxygen deficient explosives
like TNT.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
| Quote: |
Adding aluminum powder, up to a certain percentage, does normally increase the explosive power, but it also normally lowers the brisance.
|
I was planning to add it to NG/NC mixture. Pure NG has a lot of hazards around it not need to add more.
Adding Al to NG Gel would make it more powerful but I miss how to calculate the correct ratio !
| Quote: |
Aluminum is even sometimes added to very oxygen deficient explosives like TNT
|
Oh. i thought you would say if it has excess not deficient !
Al would need oxygen ! where it would get it from ? air?
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
The TNT supplies a tremendous amount of heat energy to the aluminum and yes I think for the most part the reaction is mostly with oxygen from the air
(oxygen from water in the case of sea mines, etc).
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Aluminium will react with oxygen form O2, CO2, CO, N2O, NO, NO2 and H2O in the explosion gases but also with nitrogen N2... then it will react out of
the explosion gases with air (N2 and O2 forming AlN and Al2O3).
Owing to the limitation of the minimal Al particle size (if you go too tiny the superficial oxyd layer becomes too significative vs the active metalic
Al) then the speed of burning is limited to deflagration or low order detonation...the effect with a high explosive is like a second boost, a kind of
post combustion kick.
Al is more interesting if added in over oxygenated explosives thus with positive OB; but it is sometimes used with oxygen deficient explosives like
TNT... Tritonal is such a mix of TNT (80 to 60%) and Al powder (20 to 40%).
The TNT disperse the overheated Al powder in the air making a kind of Fuel Air Explosion because in the phase after the detonation blast the Al bruns
with the surrounding air (Nitrogen and Oxygen) generating a big fire ball; then because the air has been "condensed" into solid material AlN and
Al2O3; if you have a large enough device, you get an implosion effect (the burned oxygen and nitrogen creates a kind of vacuum that must be filled by
the surrounding air outside the second blast).
[Edited on 12-5-2015 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
| Pages:
1
..
22
23
24
25
26
27 |