Difference between revisions of "Bismuth"
Diachrynic (Talk | contribs) |
|||
| Line 283: | Line 283: | ||
Bismuth reacts with halogens to produce bismuth halides. Unlike [[bismuth trifluoride]] and [[bismuth triiodide]], [[bismuth chloride|bismuth trichloride]] and [[bismuth pentafluoride]] rapidly hydrolyse in moist air and water. Bismuth will react with most acids, but [[oxygen]] or [[hydrogen peroxide]] has to be present to oxidize the metal. | Bismuth reacts with halogens to produce bismuth halides. Unlike [[bismuth trifluoride]] and [[bismuth triiodide]], [[bismuth chloride|bismuth trichloride]] and [[bismuth pentafluoride]] rapidly hydrolyse in moist air and water. Bismuth will react with most acids, but [[oxygen]] or [[hydrogen peroxide]] has to be present to oxidize the metal. | ||
| + | |||
| + | Bismuth, especially when powdered, will readily react with concentrated nitric acid to give a solution of bismuth nitrate and oxides of nitrogen, although heating might be required to archieve complete dissolution. | ||
| + | |||
| + | When bismuth chloride or bismuth nitrate solutions are diluted, they hydrolyze and form insoluble precipitates of bismuth oxychloride and bismuth oxynitrate respectivly. | ||
| + | |||
| + | When an iodide solution is added to a solution of bismuth, first black bismuth(III) iodide precipitates which redissolves in an excess of iodide to form orange tetraiodobismuthate(III). | ||
===Physical=== | ===Physical=== | ||
| Line 316: | Line 322: | ||
===Disposal=== | ===Disposal=== | ||
It's best to try to recycle the bismuth and avoid dumping it in the environment. | It's best to try to recycle the bismuth and avoid dumping it in the environment. | ||
| + | |||
| + | ==Gallery== | ||
| + | <gallery widths="200" position="center" columns="4" orientation="none"> | ||
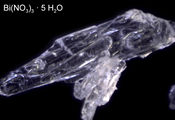
| + | Bismuth_nitrate_pentahydrate.jpg|A 3 mm long crystal of bismuth nitrate pentahydrate under the microscope | ||
| + | Bismuth_oxynitrate.jpg|When a solution of bismuth nitrate is diluted, bismuth oxynitrate precipitates | ||
| + | Tetraiodobismuthate.jpg|Bimuth solutions complex with excess iodide to orange tetraiodobismuthate | ||
| + | </gallery> | ||
==References== | ==References== | ||
Latest revision as of 22:07, 19 October 2020
 | |||||
| General properties | |||||
|---|---|---|---|---|---|
| Name, symbol | Bismuth, Bi | ||||
| Alternative name | Wismuth | ||||
| Appearance | Silvery solid, often with rainbow layer of bismuth oxide. | ||||
| Bismuth in the periodic table | |||||
| |||||
| Atomic number | 83 | ||||
| Standard atomic weight (Ar) | 208.98040 | ||||
| Group, block | , p-block | ||||
| Period | period 6 | ||||
| Electron configuration | [Xe] 4f14 5d10 6s2 6p3 | ||||
per shell | 2, 8, 18, 32, 18, 5 | ||||
| Physical properties | |||||
| Silvery, rainbow when oxidized. | |||||
| Phase | Solid | ||||
| Melting point | 544.7 K (271.5 °C, 520.7 °F) | ||||
| Boiling point | 1837 K (1564 °C, 2847 °F) | ||||
| Density at (0 °C and 101.325 kPa) | 9.78 g/cm3 g/L | ||||
| when liquid, at | 10.05 g/cm3 | ||||
| Heat of fusion | 11.30 kJ/mol | ||||
| Heat of | 179 kJ/mol | ||||
| Molar heat capacity | 25.52 J/(mol·K) | ||||
| pressure | |||||
| Atomic properties | |||||
| Oxidation states | 5, 4, 3, 2, 1, −1, −2, −3 (a mildly acidic oxide) | ||||
| Electronegativity | Pauling scale: 2.02 | ||||
| energies |
1st: 703 kJ/mol 2nd: 1610 kJ/mol 3rd: 2466 kJ/mol | ||||
| Atomic radius | empirical: 156 pm | ||||
| Covalent radius | 148±4 pm | ||||
| Van der Waals radius | 207 pm | ||||
| Miscellanea | |||||
| Crystal structure | Rhomboedral | ||||
| Speed of sound thin rod | 1790 m/s (at 20 °C) | ||||
| Thermal expansion | 13.4 µm/(m·K) (at 25 °C) | ||||
| Thermal conductivity | 7.97 W/(m·K) | ||||
| Electrical resistivity | 1.29·10-6 Ω·m (at 20 °C) | ||||
| Magnetic ordering | Diamagnetic | ||||
| Young's modulus | 32 GPa | ||||
| Shear modulus | 12 GPa | ||||
| Bulk modulus | 31 GPa | ||||
| Poisson ratio | 0.33 | ||||
| Mohs hardness | 2.25 | ||||
| Brinell hardness | 70–95 MPa | ||||
| CAS Registry Number | 7440-69-9 | ||||
| History | |||||
| Discovery | Claude François Geoffroy (1753) | ||||
Bismuth is a chemical element with symbol Bi and atomic number 83. It is a post-transition metal, grey and dense. This metal is known to be among the least toxic heavy metals. Bismuth-209 is currently known as isotope with longest decay half-time of all radioactive elements calculated to be (1.9 +/- 0.2 ) x 1019years, which is in good agreement with the theoretical prediction of 4.6 x 1019years.[1]
Contents
Properties
Chemical
Bismuth is stable to both dry and moist air at ordinary temperatures. When red-hot, it reacts with water to make bismuth trioxide[2].
- 2 Bi + 3 H2O → Bi2O3 + 3 H2
Bismuth dissolves in concentrated sulfuric acid to make bismuth(III) sulfate and sulfur dioxide.
- 2 Bi + 6 H2SO4 → 6 H2O + Bi2(SO4)3 + 3 SO2
Bismuth reacts with halogens to produce bismuth halides. Unlike bismuth trifluoride and bismuth triiodide, bismuth trichloride and bismuth pentafluoride rapidly hydrolyse in moist air and water. Bismuth will react with most acids, but oxygen or hydrogen peroxide has to be present to oxidize the metal.
Bismuth, especially when powdered, will readily react with concentrated nitric acid to give a solution of bismuth nitrate and oxides of nitrogen, although heating might be required to archieve complete dissolution.
When bismuth chloride or bismuth nitrate solutions are diluted, they hydrolyze and form insoluble precipitates of bismuth oxychloride and bismuth oxynitrate respectivly.
When an iodide solution is added to a solution of bismuth, first black bismuth(III) iodide precipitates which redissolves in an excess of iodide to form orange tetraiodobismuthate(III).
Physical
Bismuth is a brittle white-silvery metal in its pure form. It will oxidize in air to form an iridescent hue, under certain circumstances, showing many colors from yellow to blue. It has the lowest thermal conductivity of all known metals and it is the most diamagnetic pure element. Small samples of bismuth metal can levitate in the presence of strong magnetic fields. Similar to antimony, gallium, germanium and silicon, bismuth is denser in the liquid phase than the solid (like ice), expanding 3.32% on solidification.
Availability
Bismuth is present as bismuth subsalicylate in Pepto-Bismol. It can be extracted from the compound, as shown here.
Large chunks of metal can be bought as Hopper crystals, that display beautiful iridescence.
Bismuth is found in certain electronics, mostly as lead-free solder. The solder that binds the ceramic lid to the CPU appears to be mostly of bismuth, as after melting it and letting it cool in open air it displays the typical iridescence.
Some gun stores advertise shotgun pellets made of bismuth or alloys thereof as "green", or non-toxic ammo, as opposed to lead pellets.
Preparation
Bismuth can be extracted from Pepto-Bismol, by adding acid and then reducing the metal with either aluminium or other reducing metal.
Projects
- Making bismuth crystals
- Levitation with magnets
Handling
Safety
Unlike it's surrounding metals (lead, antimony, polonium), bismuth and bismuth compounds have low toxicity. Overexposure to bismuth, however, can result in the formation of a black deposit on the gums, known as a "bismuth line".
Some of its compounds, such as bismuth chloride will hydrolyze in moist air and is corrosive to skin, so protection is required when handling the compound.
Bismuth is technically radioactive; its naturally occurring isotope bismuth-209 is not fully stable. But its half-life is so high and radioactivity so low that it can be considered stable for all intents and purposes. It does not pose a radioactive hazard.
Storage
Bismuth does not require special storage. Hopper crystals can be displayed on any shelf or table.
Disposal
It's best to try to recycle the bismuth and avoid dumping it in the environment.
Gallery
References
- ↑ http://physicsworld.com/cws/article/news/2003/apr/23/bismuth-breaks-half-life-record-for-alpha-decay
- ↑ http://en.wikipedia.org/wiki/Bismuth