Difference between revisions of "Urea"
(→Physical) |
(→Reactions added barbituric acid synthesis) |
||
| Line 42: | Line 42: | ||
|- | |- | ||
| [[citric acid]] <ref>http://link.springer.com/article/10.1007/s11167-005-0579-2#page-1</ref> || || water || [[urea citrate]] || | | [[citric acid]] <ref>http://link.springer.com/article/10.1007/s11167-005-0579-2#page-1</ref> || || water || [[urea citrate]] || | ||
| + | [[malonic acid]][[sodium ethoxide]] || ||anhydrous [[ethanol]] || [[barbituric acid]] || condensation | ||
|} | |} | ||
Revision as of 01:49, 1 March 2016
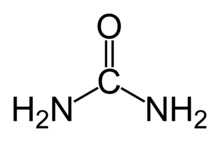
Urea or carbamide, is an organic compound commonly used as a fertilizer. It has the chemical formula (NH2)2CO. Urea was the first organic chemical produced from inorganic chemicals, a breakthrough in chemistry, which proved organic substances can be produced from inorganic substances, disproving the notion of vitalism.
Contents
Properties
Chemical
Thermal decomposition of urea produces ammonium isocyanate, who in turn yields isocyanuric acid.
- NH2CONH2 → NH4NCO → HNCO + NH3
Urea is a weak organic base. It forms salts with strong acids. Despite its two amino groups, it acts as a monoprotic base, only accepting one H+ cation. With diprotic acids such as sulfuric, urea forms two types of salts: monocarbamide dihydrosulfate (carbamonium bisulfate, analogous to inorganic bisulfates) and dicarbamide dihydrosulfate (carbamonium sulfate, analogous to inorganic sulfates).
A frequent contaminant of urea is Biuret. A high concentration of Biuret mitigates against using urea as a crop fertilizer, but makes it more valuable as an animal feed additive.
Physical
Urea is a white crystalline substance. Its melting point is between 133-135 °C. Urea is soluble in water (107.9 g/100 ml at 20 °C) and when dissolved, it is neutral on the pH scale. Urea is soluble in glycerol (500 g/L) and slightly less soluble in ethanol. Urea is soluble in dimethyl sulfoxide (40 g/100 ml at 20-30 °C, 110 g/100 ml at 90-100 °C). [1]
Availability
Urea is available as a fertilizer, either pure or mixed with other substances. It can be very cheaply purchased online, often at less than $5/kg.
ScienceCompany sells 500g of lab grade urea at 16.95 $.
Ebay has many listings for urea as a lab chemical and as fertilizer
Preparation
Urea was first synthesized by reacting silver cyanate with ammonium chloride.
- AgNCO + NH4Cl → (NH2)2CO + AgCl
Industrially it is produced by the thermal decomposition of ammonium carbamate.
It can also be produced from the reaction of phosgene and ammonia
- COCl2 + 4 NH3 → (NH2)2CO + 2 NH4Cl
Reactions
| Urea reacts with: | catalyst | solvent | product | reaction notes |
|---|---|---|---|---|
| nitric acid | water | urea nitrate | exothermic | |
| potassium nitrate [2] | hydrochloric acid | water | urea nitrate | replacement |
| hydrogen peroxide | water | urea peroxide | exothermic | |
| citric acid [3] | water | urea citrate |
malonic acidsodium ethoxide || ||anhydrous ethanol || barbituric acid || condensation |
Projects
- Urea nitrate synthesis
- Sodium azide synthesis
- Hydrazine sulfate(Hoffman Rearrangement)
- Thermal decomposition of molten urea to biuret and ammonia.
Handling
Safety
Urea is non-toxic, though is may cause irritations to sensitive tissues. It may corrode metallic surfaces, even the more resistant forms of stainless steel.
Storage
Urea doesn't require any special storage, and can be safely stored in glass, metal, plastic or ceramic containers.
Disposal
As urea is a good fertilizer, it can be safely dumped in the soil, although it's recommended to avoid dumping it in water reservoirs to prevent the growth on unwanted algae. It can also be used to as a nitrogen source for aerobic bacteria in the conversion of sawdust to compost.
References
- ↑ http://www.gaylordchemical.com/uploads/images/pdfs/literature/102B_english.pdf Gaylord Chemical Company
- ↑ Making Urea Nitrate without Nitric Acid
- ↑ http://link.springer.com/article/10.1007/s11167-005-0579-2#page-1