Difference between revisions of "Xylene"
(→Availability) |
|||
| Line 10: | Line 10: | ||
==Availability== | ==Availability== | ||
| − | Xylene is available as a paint thinner, either pure or mixed with other low chain hydrocarbons, as well as traces of [[butanol]] or [[butyl acetate]] and it's always a mixture of the three isomers. It can also be carefully removed from mixed solvents commonly found in hardware stores by fractional [[distillation]]. Individual isomers can be purchased from chemical suppliers, though they aren't cheap. Because the boiling point of the three isomers is very close, it's extremely difficult to separate the three isomers via distillation. | + | Xylene is available as a paint thinner, either pure or mixed with other low chain hydrocarbons, as well as traces of [[butanol]] or [[butyl acetate]] and it's always a mixture of the three isomers. It can also be carefully removed from mixed solvents commonly found in hardware stores by fractional [[distillation]]. Individual isomers can be purchased from chemical suppliers, though they aren't cheap. Because the boiling point of the three isomers is very close, it's extremely difficult to separate the three isomers via fractional distillation. |
==Preparation== | ==Preparation== | ||
Revision as of 17:47, 31 May 2016
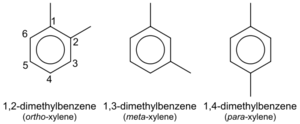
Xylene is an aromatic hydrocarbon consisting of a benzene ring with two methyl substituents. Xylene consists of three isomers: ortho-xylene, meta-xylene and para-xylene, each having a molecular formula C8H10 or, because it's an aromatic compound, C6H4(CH3)2.
Contents
Properties
Chemical
Xylene can be nitrated to give mono-, di-, trinitroxylene (TNX), using a mixture of sulfuric and nitric acid.
Physical
All three isomers of xylene are liquid at room temperature and have a characteristic paint thinner smell. The melting point of the three isomers ranges is −25 °C for o-xylene, −47.87 °C for m-xylene and 13.26 °C for p-xylene. The boiling point for each isomer is 144 °C for o-xylene, 139 °C for m-xylene and 138 °C for p-xylene. The density of each is around 0.88 g/mL for o-xylene and 0.86 g/ml for meta and p-xylene.
Availability
Xylene is available as a paint thinner, either pure or mixed with other low chain hydrocarbons, as well as traces of butanol or butyl acetate and it's always a mixture of the three isomers. It can also be carefully removed from mixed solvents commonly found in hardware stores by fractional distillation. Individual isomers can be purchased from chemical suppliers, though they aren't cheap. Because the boiling point of the three isomers is very close, it's extremely difficult to separate the three isomers via fractional distillation.
Preparation
Xylene can be prepared by methylating toluene, a process that yields all the three isomers.
Projects
- Organic extraction
- Sulfur extraction
- Tetranitroxylene synthesis
- Phthalic anhydride or phthalic acid from para-xylene
Handling
Safety
Xylene is an irritant to eyes, nose and lungs. It has low toxicity and low carcinogenic potential. Xylene is flammable and should be handled with care.
Storage
Xylene should be stored in closed bottles, away from any source of heat and light.
Disposal
Xylene can be burned, though this will generate lots of soot. Since it's an aromatic compound it's better to destroy it with Fenton's reagent.