Aerogel
 |
This article is a stub. Please help Sciencemadness Wiki by expanding it, adding pictures, and improving existing text.
|
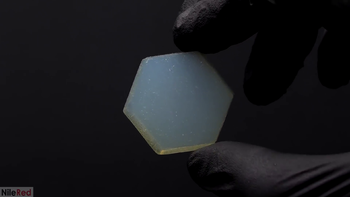
An aerogel is a synthetic porous ultralight material derived from a gel, in which the liquid component for the gel has been replaced with a gas. The result is a solid with extremely low density and extremely low thermal conductivity. Nicknames include "frozen smoke", "solid smoke", "solid air", "solid cloud", "blue smoke" (owing to its translucent nature and the way light scatters in the material). Aerogels can be made from a variety of chemical compounds.
Contents
Composition
Aerogel is a material that is 99.8% air. Aerogels have a porous solid network that contains air pockets, with the air pockets taking up the majority of space within the material. The lack of solid material allows aerogel to feel almost weightless.
The solid part of the aerogel consists generally of silicon dioxide, although aerogels obtained from alumina, chromia and tin dioxide are also known. Non-oxidic aerogels, like those made of carbon or boron nitride have also been developed.
Properties
Physical
Despite the name, aerogels are solid, rigid, and dry materials that do not resemble a gel in their physical properties: the name comes from the fact that they are made from gels.
Aerogel appear as porous solid, light blue in color, which feels fragile to touch, almost like expanded polystyrene. It is hygroscopic and will absorb fluids easily, though it may be treated to become impervious to fluids. It is an excellent heat insulator.
Chemical
Silicon dioxide aerogels can adsorb fluids. They are chemically resistant to most reagents, though HF and molten NaOH will attack them.
Availability
Aerogel is expensive and not cheap to acquire in large pieces. Smaller pieces can be bought online at more reasonable prices.
Preparation
Aerogels are produced by extracting the liquid component of a gel through supercritical drying. This allows the liquid to be slowly dried off without causing the solid matrix in the gel to collapse from capillary action, as would happen with conventional evaporation.
This method is not easy to perform by the amateur chemist, but it has been done by some people.[1]
NileRed has made a very good video on the preparation of aerogels.[2]
Uses
- Heat insulating
- Compound collecting
- Filter
- Desiccator
- Scientific demonstrations of lightweight solids
Handling
Safety
Bulk aerogel is relative harmless, though powdered particulates are irritant and
Storage
In closed boxes or on display if you just need a decorative piece.
Disposal
Generally no special disposal is required for aerogels, though it's best to mix the particulates with gypsum or bentonite, to prevent them from getting airborne.