Difference between revisions of "Onchidal"
(→Relevant Sciencemadness threads) |
|||
| Line 51: | Line 51: | ||
| BoilingPtC = | | BoilingPtC = | ||
| BoilingPt_ref = | | BoilingPt_ref = | ||
| − | | BoilingPt_notes = | + | | BoilingPt_notes = Unknown |
| Density = | | Density = | ||
| Formula = C<sub>17</sub>H<sub>24</sub>O<sub>3</sub> | | Formula = C<sub>17</sub>H<sub>24</sub>O<sub>3</sub> | ||
| Line 60: | Line 60: | ||
| MeltingPtC = | | MeltingPtC = | ||
| MeltingPt_ref = | | MeltingPt_ref = | ||
| − | | MeltingPt_notes = | + | | MeltingPt_notes = Unknown |
| − | | Odor = | + | | Odor = Unknown |
| pKa = | | pKa = | ||
| pKb = | | pKb = | ||
| Line 92: | Line 92: | ||
| ExternalMSDS = None | | ExternalMSDS = None | ||
| FlashPt = | | FlashPt = | ||
| − | | LD50 = | + | | LD50 = Unknown |
| LC50 = | | LC50 = | ||
| MainHazards = Toxic | | MainHazards = Toxic | ||
| Line 114: | Line 114: | ||
Onchidal acts as an irreversible acetylcholinesterase inhibitor, the same mechanism of action as that of the more known deadly nerve agents, which makes this compound a potent neurotoxin. However onchidal is not an organophosphorus or carbamate compound and bears little resemblance to other compounds of this nature.<ref>[https://molpharm.aspetjournals.org/content/36/3/349 Abramson SN, Radic Z, Manker D, Faulkner DJ, Taylor P (September 1989). "Onchidal: a naturally occurring irreversible inhibitor of acetylcholinesterase with a novel mechanism of action". Molecular Pharmacology. 36 (3): 349–54]</ref> | Onchidal acts as an irreversible acetylcholinesterase inhibitor, the same mechanism of action as that of the more known deadly nerve agents, which makes this compound a potent neurotoxin. However onchidal is not an organophosphorus or carbamate compound and bears little resemblance to other compounds of this nature.<ref>[https://molpharm.aspetjournals.org/content/36/3/349 Abramson SN, Radic Z, Manker D, Faulkner DJ, Taylor P (September 1989). "Onchidal: a naturally occurring irreversible inhibitor of acetylcholinesterase with a novel mechanism of action". Molecular Pharmacology. 36 (3): 349–54]</ref> | ||
| − | Ozonolysis of onchidal in [[ethyl acetate]] solution at -70 °C, followed by a reduction with [[zinc]] powder in [[acetic acid]] gave a keto-aldehyde.<ref>[https://www.sciencedirect.com/science/article/abs/pii/0045206878900433 Ireland; Faulkner; Bioorganic Chemistry; vol. 7; (1978); p. 125,129]</ref> | + | Ozonolysis of onchidal in [[ethyl acetate]] solution at -70 °C, followed by a reduction with [[zinc]] powder in [[acetic acid]] gave a keto-aldehyde.<ref name="ireland">[https://www.sciencedirect.com/science/article/abs/pii/0045206878900433 Ireland; Faulkner; Bioorganic Chemistry; vol. 7; (1978); p. 125,129]</ref> |
===Physical=== | ===Physical=== | ||
| Line 120: | Line 120: | ||
==Availability== | ==Availability== | ||
| − | Onchidal can be found in the defensive secretion of the Onchidella binneyi sea slug. Its extraction from these secretions has been described in literature.<ref | + | Onchidal can be found in the defensive secretion of the Onchidella binneyi sea slug. Its extraction from these secretions has been described in literature.<ref name="ireland" /><ref>[https://www.beilstein-journals.org/bjoc/content/pdf/1860-5397-14-197.pdf Cadelis, Melissa M.; Copp, Brent R.; Beilstein Journal of Organic Chemistry; vol. 14; (2018); p. 2229 - 2235]</ref> |
However, given that the amount of defense secretions produced by a single Onchidella slug is small, any attempt to obtain practical amounts of this chemical compound would be quite an intensive task. | However, given that the amount of defense secretions produced by a single Onchidella slug is small, any attempt to obtain practical amounts of this chemical compound would be quite an intensive task. | ||
| Line 136: | Line 136: | ||
Onchidal is a cholinesterase or acetylcholinesterase (AChE) inhibitor. A cholinesterase inhibitor (or 'anticholinesterase') suppresses the action of acetylcholinesterase. Because of its essential function, chemicals that interfere with the action of acetylcholinesterase are potent neurotoxins, causing excessive salivation and eye-watering in low doses, followed by muscle spasms and ultimately death. In case of serious exposure, [[atropine]] and/or pralidoxime are used as antidotes. | Onchidal is a cholinesterase or acetylcholinesterase (AChE) inhibitor. A cholinesterase inhibitor (or 'anticholinesterase') suppresses the action of acetylcholinesterase. Because of its essential function, chemicals that interfere with the action of acetylcholinesterase are potent neurotoxins, causing excessive salivation and eye-watering in low doses, followed by muscle spasms and ultimately death. In case of serious exposure, [[atropine]] and/or pralidoxime are used as antidotes. | ||
| − | There are not reported cases of onchidal poisonings in humans as of yet, and its toxicity to humans is unknown, thus no LD50 is available. | + | There are not reported cases of onchidal poisonings in humans as of yet, and its toxicity to humans is unknown, thus no LD50 value is available. |
Onchidal has been shown to posses anti-microbial properties, being capable of inhibiting the growth of Staphylococcus aureus. The minimum inhibitory concentration has been determined to be between 0.21 and 0.63 µg/ml, indicating that onchidal is a potent inhibitor of gram-positive bacteria. It also appears to display anti-tumoral properties, being capable of inhibiting the growth of various types of cancer tumors.<ref>[https://onlinelibrary.wiley.com/doi/abs/10.1002/cbdv.202000580 Wang, Bowen; Chen, Deli; Yu, Meng; Liu, Yangyang; Liu, Pinghuai; Zhang, Xiaopo (17 November 2020). "A Review on Metabolites from Onchidium Genus: Chemistry and Bioactivity"]</ref> | Onchidal has been shown to posses anti-microbial properties, being capable of inhibiting the growth of Staphylococcus aureus. The minimum inhibitory concentration has been determined to be between 0.21 and 0.63 µg/ml, indicating that onchidal is a potent inhibitor of gram-positive bacteria. It also appears to display anti-tumoral properties, being capable of inhibiting the growth of various types of cancer tumors.<ref>[https://onlinelibrary.wiley.com/doi/abs/10.1002/cbdv.202000580 Wang, Bowen; Chen, Deli; Yu, Meng; Liu, Yangyang; Liu, Pinghuai; Zhang, Xiaopo (17 November 2020). "A Review on Metabolites from Onchidium Genus: Chemistry and Bioactivity"]</ref> | ||
Latest revision as of 19:56, 24 October 2023
| |
| Names | |
|---|---|
| IUPAC name
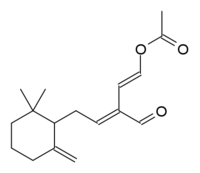
(1E,3E)-5-(2,2-Dimethyl-6-methylenecyclohexyl)-3-formylpenta-1,3-dien-1-yl acetate
| |
| Properties | |
| C17H24O3 | |
| Molar mass | 276.376 g/mol |
| Appearance | Possibly solid |
| Odor | Unknown |
| Melting point | Unknown |
| Boiling point | Unknown |
| Hazards | |
| Safety data sheet | None |
| Lethal dose or concentration (LD, LC): | |
| LD50 (Median dose)
|
Unknown |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Onchidal is a naturally occurring neurotoxin produced as a defensive secretion by the mollusc Onchidella binneyi and several other related species in Onchidella, a genus of small, air-breathing sea slugs. The compound shows promise in medicine.
Contents
Properties
Chemical
Onchidal acts as an irreversible acetylcholinesterase inhibitor, the same mechanism of action as that of the more known deadly nerve agents, which makes this compound a potent neurotoxin. However onchidal is not an organophosphorus or carbamate compound and bears little resemblance to other compounds of this nature.[1]
Ozonolysis of onchidal in ethyl acetate solution at -70 °C, followed by a reduction with zinc powder in acetic acid gave a keto-aldehyde.[2]
Physical
There is little data regarding the physical properties of onchidal.
Availability
Onchidal can be found in the defensive secretion of the Onchidella binneyi sea slug. Its extraction from these secretions has been described in literature.[2][3]
However, given that the amount of defense secretions produced by a single Onchidella slug is small, any attempt to obtain practical amounts of this chemical compound would be quite an intensive task.
Preparation
The total synthesis of this compound has not been achieved so far.
Projects
- Anti-microbial compound
- Anti-tumoral properties
- Compound collecting
Handling
Safety
Onchidal is a cholinesterase or acetylcholinesterase (AChE) inhibitor. A cholinesterase inhibitor (or 'anticholinesterase') suppresses the action of acetylcholinesterase. Because of its essential function, chemicals that interfere with the action of acetylcholinesterase are potent neurotoxins, causing excessive salivation and eye-watering in low doses, followed by muscle spasms and ultimately death. In case of serious exposure, atropine and/or pralidoxime are used as antidotes.
There are not reported cases of onchidal poisonings in humans as of yet, and its toxicity to humans is unknown, thus no LD50 value is available.
Onchidal has been shown to posses anti-microbial properties, being capable of inhibiting the growth of Staphylococcus aureus. The minimum inhibitory concentration has been determined to be between 0.21 and 0.63 µg/ml, indicating that onchidal is a potent inhibitor of gram-positive bacteria. It also appears to display anti-tumoral properties, being capable of inhibiting the growth of various types of cancer tumors.[4]
Storage
In airtight sealed glass ampoules. Keep only minute amounts.
Disposal
Can be destroyed with a strong oxidizing solution, like piranha solution.
References
- ↑ Abramson SN, Radic Z, Manker D, Faulkner DJ, Taylor P (September 1989). "Onchidal: a naturally occurring irreversible inhibitor of acetylcholinesterase with a novel mechanism of action". Molecular Pharmacology. 36 (3): 349–54
- ↑ 2.0 2.1 Ireland; Faulkner; Bioorganic Chemistry; vol. 7; (1978); p. 125,129
- ↑ Cadelis, Melissa M.; Copp, Brent R.; Beilstein Journal of Organic Chemistry; vol. 14; (2018); p. 2229 - 2235
- ↑ Wang, Bowen; Chen, Deli; Yu, Meng; Liu, Yangyang; Liu, Pinghuai; Zhang, Xiaopo (17 November 2020). "A Review on Metabolites from Onchidium Genus: Chemistry and Bioactivity"