Difference between revisions of "Tetrachlorocuprate"
From Sciencemadness Wiki
(Created page with "{{stub}} Tetrachlorocuprate structure The tetrachlorocuprate ion is a chloro complex of copper, represented by t...") |
|||
| (7 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{stub}} | {{stub}} | ||
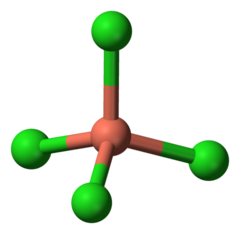
| − | [[File:Tetrachlorocuprate.png|thumb|240px|Tetrachlorocuprate structure]] | + | [[File:Tetrachlorocuprate structure.png|thumb|240px|Tetrachlorocuprate structure]] |
| − | The tetrachlorocuprate ion is a | + | The tetrachlorocuprate ion is a chloro complex of copper, represented by the formula '''[CuCl<sub>4</sub>]<sup>2-</sup>'''. |
| + | |||
==Properties== | ==Properties== | ||
The tetrachlorocuprate ion is hard to get in solid form, but is rather easy to calcinate. Tetrachlorocuprate ions react violently with [[aluminium]] to form fine [[copper]] and aluminium(III) ions. In solution, the tetrachlorocuprate ion is bright green. | The tetrachlorocuprate ion is hard to get in solid form, but is rather easy to calcinate. Tetrachlorocuprate ions react violently with [[aluminium]] to form fine [[copper]] and aluminium(III) ions. In solution, the tetrachlorocuprate ion is bright green. | ||
| + | |||
==Production== | ==Production== | ||
| − | + | This complex is generally produced by the addition of copper (II) ions to chloride ions. One easy method is by ading copper metal to a solution of [[hydrochloric acid]] and [[hydrogen peroxide]], which forms [[tetrachlorocupric acid]], the acid of the tetrachlorocuprate ion. | |
| + | |||
| + | ==Projects== | ||
| + | *PCB etchant | ||
| + | *Make coordination complexes | ||
| + | |||
| + | ==Gallery== | ||
| + | <gallery widths="200" position="center" columns="4" orientation="none"> | ||
| + | Tetrachlorocupric acid with copper by Zts16.jpg|Reaction of copper, hydrochloric acid and hydrogen peroxide, showing the distinct green color of tetrachlorocuprate. | ||
| + | </gallery> | ||
| + | |||
| + | |||
| + | ==References== | ||
| + | <references/> | ||
| + | ===Relevant Sciencemadness threads=== | ||
| + | *[http://www.sciencemadness.org/talk/viewthread.php?tid=30117 Thermochromic Tetrachlorocuprate] | ||
| + | |||
| + | [[Category:Chemical compounds]] | ||
| + | [[Category:Inorganic compounds]] | ||
| + | [[Category:Copper compounds]] | ||
| + | [[Category:Coordination complexes]] | ||
| + | [[Category:Copper complexes]] | ||
| + | [[Category:Acids]] | ||
| + | [[Category:Anions]] | ||
| + | [[Category:Polyatomic ions]] | ||
| + | [[Category:Materials stable only in solution]] | ||
Latest revision as of 20:16, 26 December 2022
 |
This article is a stub. Please help Sciencemadness Wiki by expanding it, adding pictures, and improving existing text.
|
The tetrachlorocuprate ion is a chloro complex of copper, represented by the formula [CuCl4]2-.
Contents
Properties
The tetrachlorocuprate ion is hard to get in solid form, but is rather easy to calcinate. Tetrachlorocuprate ions react violently with aluminium to form fine copper and aluminium(III) ions. In solution, the tetrachlorocuprate ion is bright green.
Production
This complex is generally produced by the addition of copper (II) ions to chloride ions. One easy method is by ading copper metal to a solution of hydrochloric acid and hydrogen peroxide, which forms tetrachlorocupric acid, the acid of the tetrachlorocuprate ion.
Projects
- PCB etchant
- Make coordination complexes
Gallery