| Pages:
1
2 |
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Chlorination of Sulphur
deltaH's thread in Energetics mentioned 'Factice', which led me to attempt to make Brown Factice.
To make White factice, the papers say that Sulphur Chloride SCl2 is used instead of elemental Sulphur, so here is my second effort at
SCl2 synthesis.
From http://en.wikipedia.org/wiki/Sulfur_dichloride it appears that the reaction is multi-step and complicated, however the process is simply a matter
of passing enough Chlorine gas over powdered elemental Sulphur.
Here's what i came up with :-

In the vacuum flask on the left is 25g KMnO4 with an addition funnel on top, containing 100ml of 20% HCl, acting as the Cl2
generator.
2 KMnO4 + 16 HCl => 2 KCl + 2 MnCl2 + 5 Cl2 + 8 H2O
The gas is led to one side of the U-tube, at the bottom of which is 5g powdered Sulphur and tiny magnetic stir-bar.
To dispose of excess Chlorine gas the right hand outlet is led into a 'scrubber' filled with NaOH solution.
2 Cl2 + 2 NaOH => NaCl + NaClO + H2O.
The HCl flow was begun at ~1 drip per second, and remained there for the entire run.
At the start, the Sulphur looked like this :-

After 5 mins :-

At 8 mins 18 seconds it was all a liquid. Note the distinct surface layer :-

At 30 minutes the liquid was very much Red. Notice the volume increase :-
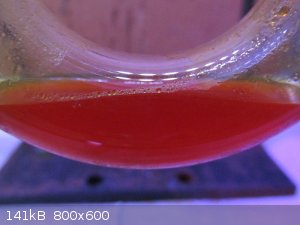
Amazingly the bubbling of the scrubber pot could be affected by agitating the liquid at this stage.
So much so that sufficient agitation caused a slow suck-back effect.
Wagging a magnet under the stir-bar was so effective that i got carried away, causing a lot of suck-back and destruction of the product.

In the future i must either grow up and take things seriously (unlikely) or make an anti-suck-back jar (much more likely).
Edit: typos, equations and balancing errors corrected (cheers blogfast25)
[Edited on 24-3-2015 by aga]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Quote: Originally posted by aga  |
The gas is led to one side of the U-tube, at the bottom of which is 5g powdered Sulphur and tiny magnetic stir-bar.
|
Ingenious.
I have made S2Br2 several times. This is easy, but never SCl2. IIRC Len1 has a procedure for making it in Prepublication. You have probably seen it
I imagine.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
I also really like that setup.
|
|
|
Molecular Manipulations
Hazard to Others
  
Posts: 447
Registered: 17-12-2014
Location: The Garden of Eden
Member Is Offline
Mood: High on forbidden fruit
|
|
Nice job man!
Keep in mind that this stuff is rather unstable, and exists in equilibrium with both disulfur dichloride and sulfur tetrachloride (somehow both,
which is weird).
At first when chlorination begins this reaction predominates:
2 S + Cl2 → S2Cl2, once all or most of the sulfur is in the first oxidation state, it oxidizes again to form
sulfur dichloride, which at low temperatures can be further oxidized to the tetrachloride, which is quite unstable and only a small fraction will form
regardless of excess chlorine being added. Sulfur dichloride slowly releases chlorine forming disulfur dichloride again, so it's best to store it in a
freezer.
I'm going to use your setup next time I try the reaction. In the past I've used a pressure equalized addition funnel a two neck flask and a Liebig
condenser to distill it as it's produced. What happens is rather than boiling it decomposes to disulfur dichloride which boils at 137.1 °C, I used a
big excess of chlorine, which reacts again as shown above to form the pure sulfur dichloride. I have one ampule of the stuff left, and every time I
break one to use it, pressure is released and chlorine can be detected via my usual (smell, cough, run, throw up, repete).
I like your setup cause it doesn't require distillation (and I broke my damned Liebigs, both!). However the sulfur I have is only 90% pure, the rest
being bentonite clay, so that won't work...
Anyway great job!
-The manipulator
We are all here on earth to help others; what on earth the others are here for I don't know. -W. H. Auden
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
Keep it away from ethylene!
|
|
|
Molecular Manipulations
Hazard to Others
  
Posts: 447
Registered: 17-12-2014
Location: The Garden of Eden
Member Is Offline
Mood: High on forbidden fruit
|
|
Sulfur dichloride reacts with ethyene? I know disulfur dichloride combines to produced bis(2-chloroethyl) sulfide (mustard gas) but does sulfur
dichloride work as well?
The reaction for the former is: S2Cl2 + 2 C2H4 → (ClC2H4)2S + S, so
this could be applied to sulfur dichloride like this: SCl2 + 2 C2H4 → (ClC2H4)2S.
But just because I can balance a reaction doesn't mean it works, do you have a reference?
-The manipulator
We are all here on earth to help others; what on earth the others are here for I don't know. -W. H. Auden
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Wow aga, that is extremely beautiful! Well done 
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Very nice experiment. I like direct union of elements reactions.
Shame about the suck back, though. A suck back vessel would have saved it  . .
Also, you used KMnO<sub>4</sub>(permanganate +7), not KMnO<sub>3</sub> (manganate +5).
And, NaOH + plus chlorine leads to NaClO, not NaClO<sub>3</sub>...
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Thanks for the comments !
It's a breeze to do, and i shall do it again (properly) in a day or two to get some SCl2 for the White Factice experiment.
Quote: Originally posted by blogfast25  | Also, you used KMnO<sub>4</sub>(permanganate +7), not KMnO<sub>3</sub> (manganate +5).
And, NaOH + plus chlorine leads to NaClO, not NaClO<sub>3</sub>...
|
Doh ! KMnO3 was a typo.
NaClO3 was my own lazy self not checking the reaction at all, and copying it wholesale off the 'web.
Thanks for noticing and for the corrections.
I should mention that the overall reaction is slightly exothermic, yet starting with 5g of S never made the glass too hot to touch.
[Edited on 24-3-2015 by aga]
[Edited on 24-3-2015 by aga]
|
|
|
Molecular Manipulations
Hazard to Others
  
Posts: 447
Registered: 17-12-2014
Location: The Garden of Eden
Member Is Offline
Mood: High on forbidden fruit
|
|
I melt the sulfur first and then add chlorine, either works, but I think yields will be better if it starts out molten in your' setup, but with mine
it shouldn't make any difference (because the apparatus is closed off).
Depending on the temperature of the hydroxide solution, chlorate can form, I believe hypochlorite decomposes at around 70°C.
-The manipulator
We are all here on earth to help others; what on earth the others are here for I don't know. -W. H. Auden
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
Quote: Originally posted by Molecular Manipulations  | Sulfur dichloride reacts with ethyene? I know disulfur dichloride combines to produced bis(2-chloroethyl) sulfide (mustard gas) but does sulfur
dichloride work as well?
The reaction for the former is: S2Cl2 + 2 C2H4 → (ClC2H4)2S + S, so
this could be applied to sulfur dichloride like this: SCl2 + 2 C2H4 → (ClC2H4)2S.
But just because I can balance a reaction doesn't mean it works, do you have a reference? |
I believe the SCl2 will be contaminated with S2Cl2.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
It will, however an over-dose of Chlorine will make the product predominantly SCl2 initially, decomposing over time to
S2Cl2.
It seems that sealing the container ends up causing a slight positive pressure of chlorine gas, halting the decomposition effect.
|
|
|
Pyro
International Hazard
    
Posts: 1305
Registered: 6-4-2012
Location: Gent, Belgium
Member Is Offline
Mood: No Mood
|
|
MM, don't you have a hell of a time cleaning the flask?
Do you distill the S2Cl2 off immediately?
all above information is intellectual property of Pyro.  |
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Quote: Originally posted by Pyro  | MM, don't you have a hell of a time cleaning the flask?
Do you distill the S2Cl2 off immediately? |
As you can see, the suck-back destroyed the product, and i've left it all in the fume hood overnight for the Cl2 to disappear.
Everyone thinks we're about to re-open our pool 3 months early ...
The previous run ended up with clag-free SCl2 after filtering, and there was no real meass to speak of.
I stored it in a handy plastic container overnight, which the SCl2 ate through, and ran away to freedom (hence this second effort).
Edit:
Sorry, i thought you meant Mmmm instead of Molecular Manipulations.
[Edited on 24-3-2015 by aga]
|
|
|
Molecular Manipulations
Hazard to Others
  
Posts: 447
Registered: 17-12-2014
Location: The Garden of Eden
Member Is Offline
Mood: High on forbidden fruit
|
|
Ever melted rubber in glassware? It's easier to buy a new flask then clean the shit.
Since I can't afford such things, I cleaned it out yes. I melted it and poured out what was left, let it cool and scraped out some more, added nitric
acid, sulfuric acid with hydrogen peroxide and finally toluene to get most of it out. The sulfur I think wasn't the hard part, toluene would taken
care of that from the start, it was then damn clay.
Yeah I had distillation going at the time of the reaction. Otherwise it would have just decomposed to disulfur dichloride.
-The manipulator
We are all here on earth to help others; what on earth the others are here for I don't know. -W. H. Auden
|
|
|
Pyro
International Hazard
    
Posts: 1305
Registered: 6-4-2012
Location: Gent, Belgium
Member Is Offline
Mood: No Mood
|
|
Sounds nasty! should try hot chromic acid as per Vogel's prescription 
I'm trying this as soon as I get my lab relocated!
all above information is intellectual property of Pyro.  |
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
I tried Making factice artificial rubber in two 250ml beakers, and it didn't work.
The beakers were horribly gooped up with brown tar.
Toulene hardly touched it, nor did acetone.
Pihraña Fluid was a thought, although my H2O2 is just 3%.
A few hours in a charcoal fire removed all of the brown goop, but also removed the graduations, which i liked.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
I hadn't seen it as it happens.
Having now looked at it, all i have done is pass chlorine over sulphur powder and got lucky !
Len1's Work is rather more involved, precise and useful than my random experiment.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Yes, it is easy to get over awed by Len1's accomplishments. He is after all a professor at a university in Australia. When doing the experiments for
his book I believe he had full access to all of his school's labs, library resources, colleagues, etc. This is not to take away from his hard work
and brilliant mind.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
My 1 hour's tinkering, a few photos and a final failure due to over-excited idiocy really bear no comparison to Len1's paper.
Having said that, it seems to work, and is do-able for an amateur.
|
|
|
Oscilllator
National Hazard
   
Posts: 659
Registered: 8-10-2012
Location: The aqueous layer
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Magpie  |
Yes, it is easy to get over awed by Len1's accomplishments. He is after all a professor at a university in Australia. When doing the experiments for
his book I believe he had full access to all of his school's labs, library resources, colleagues, etc. This is not to take away from his hard work
and brilliant mind. |
Do you know which university he is a professor at? I am currently sitting in a lecture hall at a university in Australia 
Quote: Originally posted by Pyro  | MM, don't you have a hell of a time cleaning the flask?
Do you distill the S2Cl2 off immediately? |
Cleaning the flask was actually really easy (much to my surprise). All you need to do is get a hot dilute solution of NaOH and the sulfur comes right
off leaving the flask sparkling clean. I have some cool pictures of the reaction of sulfur and chlorine, perhaps I'll post them when I get home.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Quote: Originally posted by Oscilllator  |
Do you know which university he is a professor at? I am currently sitting in a lecture hall at a university in Australia  |
Flinders University, Adelaide
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Oscilllator
National Hazard
   
Posts: 659
Registered: 8-10-2012
Location: The aqueous layer
Member Is Offline
Mood: No Mood
|
|
To bad, I'm at the university of new south wales 
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Success !
Tried again with an anti-suck-back device and some brains engaged.
Result is 9.38g, 6ml of stuff, having started as 5.25g of flowers of Sulphur.
Woohoo !

|
|
|
Molecular Manipulations
Hazard to Others
  
Posts: 447
Registered: 17-12-2014
Location: The Garden of Eden
Member Is Offline
Mood: High on forbidden fruit
|
|
Nice, 31% yield ain't bad for such a simple apparatus.
-The manipulator
We are all here on earth to help others; what on earth the others are here for I don't know. -W. H. Auden
|
|
|
| Pages:
1
2 |