Maximum effort.
The 2,5-dimethoxy-4-(alkylthio)benzaldehydes
212.27 g/mol (R = methyl)
226.30 g/mol (R = ethyl)
240.32 g/mol (R = 1-propyl)

Historically, the formation of the 4-alkylthio substituent has been regarded as a pick-and-mix of excessively
difficult, prohibitively hazardous, and/or atrociously malodorous procedures by the amateur chemist, leaving most with no other option than to give up
the chase. At the same time, however, the technology to overcome these barriers has been out there, waiting to be commonly accepted and
adopted through experimental rediscovery. [1][2] Such an initiative was eventually
taken by the Sciencemadness user Ullmann, whose absolute treasure chest of a thread [3]
I would have initially overlooked, were it not for a chance encounter with a more recent thread [4] in which another Sciencemadness user, turd, has done an outstanding job in presenting the core issues, and the ways in
which Alexander Shulgin, Ullmann, and later they themselves tackled them. Without these contributions, my own efforts in this area would exist only in
my dreams.
This post comprises my darnedest efforts to compile the entire diverging, 11-step sulfur chemistry portion of the overall endeavor
into a concise narrative whose progression remains faithful to the staged approach, according to which the actual events unfolded and interconnected
observations were made: in brief, a thiophenolic precursor was prepared, and divided into three portions (excluding an analytical sample), two of
which were selectively alkylated at the sulfur prior to stages of jugate permethylation and formylation with the third portion.
Even though some of the materials did sport a foul odor — mostly when impure — none were anywhere near as bad in terms of
volatility or detection threshold, or even in the qualitative sense, as the simple thiols (encountered in alternative approaches) are purported to be.
Honorable mentions should, however, be given out to a very impure sample of 2,5-dimethoxy-4-(methylthio)benzaldehyde, as well as a supposedly
microbial smell which haunted the immediate vicinity of the sink where glassware was cleaned; shortly after trace amounts of several of the
organosulfur materials were flushed down the drain, the exact same malodor would appear, and linger for a few days. Previously unknown, I would
describe the aroma as being reminiscent of decaying organic matter, and decidedly sewer-like. Interestingly, although the phenomenon certainly seemed
to coincide with the handling of everything from the thiophenol all the way to the methoxybenzenes (at least), disposal of several milligrams of the
five-month-old thiophenol down the same (interveningly inactive) sink caused no odors whatsoever.
Finally, this sequence of syntheses spans the breakage of a K-type thermometer and two thermocouples, which (along with some strange
values) is why, in December, I redetermined several of the melting point values — while also determining a few missing ones. This was done in one
session, using a thermocouple whose readings are reliable, albeit depressed by ~1°C (depending on the temperature range). Although the materials had
been stored for about 4–5 months (in airtight containers, at room temperature, and mostly protected from UV), they didn't appear to have degraded
significantly: only some rather superficial darkening was observed on the sample of propylthiohydroquinone. The verified values are given in
parentheses after the original values (not to be confused with literature values), and may be narrower due to a slower incrementation of temperature.
[1]: Lau & Kestner: Synthesis of 5-hydroxy-1,3-benzoxathiol-2-ones. https://doi.org/10.1021/jo01276a025
[2]: PiHKAL: #167 4T-MMDA-2. http://isomerdesign.com/PiHKAL/read.php?id=167
[3]: (Sciencemadness) Thread by Ullmann. https://www.sciencemadness.org/whisper/viewthread.php?tid=11...
[4]: (Sciencemadness) Thread by turd. https://www.sciencemadness.org/whisper/viewthread.php?tid=62...
Chapter I
THIOPHENOL
[a2] 1,4-benzoquinone
108.10 g/mol
Since the springtime experiments, I felt that I had unearthed sufficient evidence against water to revert back to using an alcoholic solvent. Because
I was able to conclude that my rather dilute hydrogen peroxide was definitely capable of producing benzoquinone, I prioritized improving the other
parameters over obtaining a higher concentration or seeking a different oxidizer altogether. Eventually, a striking experimental report by the
Sciencemadness user homeslice
[1] convinced me to trial the profound modification of
bringing the complete reaction mixture to a boil for just 2–3 minutes. Even though this approach seemed to contradict my previous experimental
findings somewhat, it did prove exceedingly efficient.
[1]: (Sciencemadness) homeslice: Experimental report. https://www.sciencemadness.org/whisper/viewthread.php?tid=14...
Experiment 3 (-ish)
In a 1000 mL Erlenmeyer, hydroquinone (40.30 g, 366 mmol) and iodine (0.65 g, 2.6 mmol) were dissolved in isopropyl alcohol (150 mL) with mild heating
and magnetic stirring. There was then added, in one portion, 11.9% hydrogen peroxide (140 g, 489 mmol), after which the mixture was promptly heated to
its boiling point, maintaining reflux for two minutes under a 200 mm Liebig condenser
[1] before
cessation of the heating. The mixture was stirred for another 10 minutes on the synchronously cooling hotplate prior to measuring its temperature at
~71°C (using IR); observing no more liberation of oxygen; and moving the flask onto a lab jack, where it was allowed to cool to 50°C over 20
minutes. Further cooling was effected by placing the flask in a cold water bath, where an abundant crystal formation was observed after 30 minutes.
[1]: The condenser was fitted with a U-bend (made up of adapters), whose purpose was to direct any spillage into an empty 500 mL
flask in the event of a runaway reaction. No boil-over occurred, but the vigorous evolution of oxygen did enable approximately a gram of
quinone-containing solvent vapor to make it past the condenser.
[Fig. 1] Iodine and hydroquinone under isopropanol
 [Fig. 2] Complete reaction mixture on the verge of boiling
[Fig. 2] Complete reaction mixture on the verge of boiling
 [Fig. 3] Above mixture about one minute later
[Fig. 3] Above mixture about one minute later
 [Fig. 4] Post-reaction mixture cooling down
[Fig. 4] Post-reaction mixture cooling down
 Work-up
Work-up
The crystal-laden flask was kept in the freezer for 90 minutes prior to vacuum filtering the mixture and rinsing the obtained solids with 5 g of
i-PrOH. Then, once the filter cake had been compressed and suctioned free of most of the alcohol, it was recrystallized from 35 g of
i-PrOH. The filtered crystals were placed (outside) in front of a fan in a crystallizing dish, where they were dried (with occasional mixing)
over about two hours. These crystals, while considerably cleaner, retained a rather dark overall shade of yellow, with a generous sprinkling of ones
that were outright reddish; a melting point of 115.5–117.7°C (lit. 115–116°C) was determined.
[1] The material was placed in a brass mortar where it was triturated somewhat crudely under 35 g of
i-PrOH.
Filtration of the mixture gave a slightly reddish liquor, and a high return
[2] of a crystalline
powder which still appeared quite dirty. The solid was air-dried as before, and then recrystallized once more from 75 g of
i-PrOH to little
apparent avail; after cooling the mixture in the refrigerator, vacuum filtering it, and washing the filter cake using 20 g of room-temperature
i-PrOH, the twice recrystallized, thrice air-dried yield of adamantly contaminated crystalline 1,4-benzoquinone was 31.14 g (78.7%).
[1]: The melting point test was expedited due to highly irritating fumes emanating from the heated capillary tube, leading to some
broadening of the experimental value.
[2]: No more than a gram of the quinone seemed to dissolve in the amount of (room-temperature)
i-PrOH used.
[Fig. 5] Crystal formation in cooled post-reaction mixture
 [Fig. 6] Vacuum filtration to separate crude product
[Fig. 6] Vacuum filtration to separate crude product
 [Fig. 7] Separated crude product
[Fig. 7] Separated crude product
 [Fig. 8] First recrystallization; crystallized splatters of hot solution
[Fig. 8] First recrystallization; crystallized splatters of hot solution
 [Fig. 9] Recrystallized product
[Fig. 9] Recrystallized product
 [Fig. 10] Triturated product under fresh isopropanol
[Fig. 10] Triturated product under fresh isopropanol
 [Fig. 11] Second recrystallization
[Fig. 11] Second recrystallization
 [Fig. 12] Alternative perspective (after about a minute)
[Fig. 12] Alternative perspective (after about a minute)
 [b3] 5-hydroxy-1,3-benzoxathiol-2-one
[b3] 5-hydroxy-1,3-benzoxathiol-2-one
Also called: This
168.18 g/mol
Here's a
very nifty little trick, and a key feature of this approach in terms of amateur-friendliness: when 1,4-benzoquinone is treated with
an excess of thiourea in the presence of a strong acid catalyst, a thiouronium salt is formed that can then rapidly cyclize on heating to give an
imine intermediate, which in turn hydrolyzes with the loss of ammonia to give the title compound.
[1][2][3][4][5][6] Not only is this resulting heterocyclic intermediate valuable due to its convenient and high-yielding
formation and subsequent hydrolysis (c
3) — it also allows for the independent alkylation of each oxygen, paving the way to the
fascinating 2- and 5-monoethylated
tweetios, like the 5-ethoxy-4-ethylthio-2-methoxyphenylethylamine.
[6]
Going forward, it should be kept in mind that the thiophenol is remarkably sensitive to oxidation, and any handling in basic
conditions should be performed under an inert atmosphere, with care being taken to deoxygenate solvents beforehand.
[1]: Lau & Kestner: Synthesis of 5-hydroxy-1,3-benzoxathiol-2-ones. https://doi.org/10.1021/jo01276a025
[2]: PiHKAL: #167 4T-MMDA-2. http://isomerdesign.com/PiHKAL/read.php?id=167
[3]: (Sciencemadness) Thread by Ullmann. https://www.sciencemadness.org/whisper/viewthread.php?tid=11...
[4]: (Sciencemadness) Thread by turd. https://www.sciencemadness.org/whisper/viewthread.php?tid=62...
[5]: (Hyperlab) Obtaining mercaptohydroquinone. https://hyperlab.info/inv/index.php?s=&act=ST&f=17&a...
[6]: (Hyperlab) miamiechin: 2C-T-2-5EtO. https://hyperlab.info/inv/index.php?s=&act=ST&f=17&a...
Experiment 1
Onto a jointed 1000 mL Erlenmeyer flask were stacked, in order: a 200 mm Liebig condenser; a vertical vacuum distillation adapter with a length of
tubing leading to a dilute NaOH solution (gas scrubber); and a loosely stoppered 250 mL separating funnel. To a stirred solution of thiourea (23.28 g,
306 mmol) in approximately 2.5N hydrochloric acid (~244 mL, 557 mmol)
[1] in the flask there was
added, at a rapid dropwise pace from the funnel on top, a solution of 1,4-benzoquinone (30.00 g, 278 mmol) in acetic acid (170 mL). The addition was
completed in 30 minutes, and it caused the temperature of the mixture to gradually rise to around 40°C. Once 15 minutes had passed since the end of
addition, the mixture had cooled to 37°C, and solids began to precipitate. The resultant, thick suspension was stirred for as long as it took to let
it cool down to as close to room temperature as it was going to get
[2] before adding a further
portion of 33% HCl (18.50 g, 167 mmol) and then gently heating the mixture to reflux, where it was maintained until an hour had elapsed since it was
heated past 80°C.
[3]
[0]: The temperatures were measured from exterior surfaces of the glassware using IR.
[1]: Prepared from 61.56 g of 33% HCl and 192 mL of water. Estimating a normality like this (as opposed to calculating it exactly via
titration of molarity) seems about as counter-intuitive as wearing sunglasses to bed, but there you go. In fact, full disclosure: none of my acids and
bases are properly titrated (nor have they been thus far), and claims like the above
557 mmol of HCl quite frankly do a much better job at
depicting my intentions with what I've been sold than they do the exact number of millimoles employed. That said, I'm not looking to advocate undue
laxness; what we have in titration is a fantastic opportunity to improve. First thing tomorrow.
[2]: This is ambiguously expressed as "After 90 minutes [added...]" in my notes, and based on the available documentation, it's hard
to tell whether this translates to 90 or 75 minutes following the end of addition. Either way, the mixture was slow to cool, and (IIRC) barely did so
past 30°C; I believe that there was some exotherm accompanying the solid formation, and that if frictional heat from the stirring was a thing, it was
in addition to a significant heat transfer from the active (i.e. perceivably warm) hotplate beneath.
[3]: The temperature of 80°C, while arbitrarily chosen for timing the reflux, was noteworthy for being roughly the point at which
bubbles began evolving on heating; likely signifying that a reaction was taking place.
[Fig. 13] Addition of benzoquinone solution to thiourea in hydrochloric acid
 [Fig. 14] Mixture following complete addition
[Fig. 14] Mixture following complete addition
 [Fig. 15] Alternative perspective (6500K lighting)
[Fig. 15] Alternative perspective (6500K lighting)
 [Fig. 16] Initial formation of solids
[Fig. 16] Initial formation of solids
 [Fig. 17] Suspension of supposed isothiouronium chloride
[Fig. 17] Suspension of supposed isothiouronium chloride
 [Fig. 18] Alternative perspective
[Fig. 18] Alternative perspective
 Work-up
Work-up
The post-reaction mixture was set aside to cool at room temperature. Crystals began forming from the solution at >60°C, and an impressive crop was
observed after seven more stationary hours. This was stored below 10°C for some hours prior to cooling to a temperature of 0–4°C, at which the
mixture was vacuum filtered. The unrinsed filter cake
[1] was spread out over a coffee filter,
which was placed on several layers of paper towel. Air-dried, the crystals weighed 42.73 g (91.5%), melted at 177.7–179.8°C (lit.
170.5–172.5°C;
[2] 175.5–176°C
[3] ),
and had a rather pleasant, familiar odor with a low detection threshold.
[4]
[1]: Not knowing much about the solubility of this product, and deeming it quite pure by appearance, I thought it best to not risk
rinsing it. In hindsight, it would have made a lot of sense to at least do a quick rinse with water; the solubility seems negligible, and the
obnoxious evaporation of acid fumes could have been mostly avoided.
[2]: PiHKAL: #167 4T-MMDA-2. http://isomerdesign.com/PiHKAL/read.php?id=167
[3]: SpectraBase: 1,3-BENZOXATHIOL-2-ONE, 5-HYDROXY-. https://spectrabase.com/spectrum/EjXXUEBshAz
[4]: Visually undetectable quantities on crudely wiped down surfaces could be smelled clearly. The
aroma bears an uncanny similarity to that of an odorous sample of impure crystalline MDMA which I encountered many years ago; whose origin was
completely unknown; and whose odor I have come to describe as resembling of root beer (which is entirely debatable). Having said all that, it's a
distinct possibility that, just as back then, the odor belongs to an impurity, and that none of it is inherent to the actual product. Interestingly, I
later found the odor of the crude 2-hydroxy-5-methoxyacetophenone (the phenolic side product of c
1) to exhibit a nearly identical
component.
[Fig. 19] Initial crystal formation
 [Fig. 20] Above mixture after seven hours at 24°C
[Fig. 20] Above mixture after seven hours at 24°C
 [Fig. 21] Obtained 5-hydroxy-1,3-benzoxathiol-2-one (in 6500K lighting)
[Fig. 21] Obtained 5-hydroxy-1,3-benzoxathiol-2-one (in 6500K lighting)
 [c3] 2,5-dihydroxythiophenol
[c3] 2,5-dihydroxythiophenol
Also called: Mercaptohydroquinone
142.18 g/mol
In this next step, the isolated intermediate is saponified using aqueous alkali to yield the desired thiophenol. While the obtained crude yield
appeared to be nearly quantitative, the initial melting point tests indicated a significant degree of impurity. It's possible that the conversion via
this described procedure may have been incomplete, as I certainly overestimated the purity of my NaOH. On top of this, there was observed what appears
to be a tendency to discolor and degrade on heating, which I suspect to be a characteristic of the product, and thus mostly independent of the initial
impurity. Prolonged storage in solution also seems like it could be problematic. All things considered, potential improvements include additional
base, thin-layer chromatography and, weirdly enough, not bothering with further purification.
[0]: For references, see the previous step.
Experiment 1
In a two-necked 1000 mL RBF, 5-hydroxy-1,3-benzoxathiol-2-one (40.01 g, 0.24 mol) was added through a 200 mm Liebig to a magnetically stirred,
deoxygenated
[1] solution of NaOH (38.57 g, 0.96 mol) in water (320 mL), against a gentle
overpressure of argon. Maintaining the argon flow throughout, the mixture was heated under reflux for an hour and then moved to a water bath, where it
was cooled to ambient temperature prior to a colorful
[2] acidification via dropwise addition of
33% hydrochloric acid (137.20 g, 1.24 mol).
[1]: Deoxygenation was performed by pulling an aspirator vacuum over the mixture several times, each time normalizing the pressure
using argon: first for a period of 5 minutes, and then three more times for as long as it took to reach the final depth of vacuum.
[2]: Green → gray → red amber (with initial foaming) → green → gray → nearly white with a green tinge, and opaque from
precipitation and effervescence.
[Fig. 22] Dissolution of starting ester in aqueous alkali
 [Fig. 23] Above mixture after about two minutes
[Fig. 23] Above mixture after about two minutes
 [Fig. 24] "Red amber" stage of post-reaction acidification
[Fig. 24] "Red amber" stage of post-reaction acidification
 [Fig. 25] Acidified mixture
[Fig. 25] Acidified mixture
 Work-up
Work-up
The acidified suspension was subjected to three vacuum-argon cycles, each giving rise (and fall) to a transient, pastry-like crust over an abundant
liberation of residual (hydrogen sulfide-smelling) gas. The mixture was then vacuum filtered to obtain a portion of solid material with a dirty white
color and a melting point of 109–116°C (lit. 118–119°C
[1] ). Extraction of the filtrate
using ethyl acetate, followed by evaporation to dryness, gave a comparable amount of solid which was visibly cleaner and melted at 118–120°C. The
portions were combined (thoughtlessly, before knowing their melting points) for a crude yield of 33.19 g (98.1%).
Recrystallization of the crude product was explored. An unrecorded portion (presumably everything) was dissolved in 60 g of boiling ethyl acetate, and
20 g of heptane was added with the intention of lowering its solubility, but the liquids were immiscible. An initial crop of finer off-white crystals
(12.8 g, MP ~114°C) was obtained, followed by a second crop (10.9 g) of larger, more distinct crystals with a green hue from concentration of the
filtrate through distillation.
Evaporation of the concentrated mother liquor gave about six grams of a gooey, green, crystalline mass, from whence an extraction of product was
attempted with two portions of boiling toluene (25, then 21 g) that were decanted off of a sunken brown oil. The combined extracts were heated
together to redissolve everything. On cooling, a light-brown oil separated before the formation of crystals. A gram of
n-PrOH was added in an
attempt to keep more of the oil in solution; the oil temporarily dissolved, but reappeared at a lower temperature, coinciding with crystal formation
and prompting the addition of another gram of
n-PrOH. The mixture was heated to redissolve the crystals, and on cooling, the oil initially
remained in solution while crystals formed. At 24°C, however, some oil had still separated. The mixture was placed in a freezer and the cold liquor
was decanted off. Residual oil and solvent were removed by pressing the solids between layers of paper towel before air-drying them to obtain 2.57 g
of cream-colored crystals which melted at about 106°C.
The 10.9 g portion was recrystallized from 32 grams of aqueous 10% methanol to yield 8.09 g of crystals (with a melting point of ~117°C) via vacuum
filtration of the refrigerated (<10°C) mixture. The mother liquor was used to dissolve the residue from evaporation of the above toluene, as well
as the cream-colored crystals obtained from it, and the solution was allowed to slowly evaporate from a cling-film-covered beaker for about seven
weeks; the remaining liquid was then pipetted off, leaving yellow, crystalline solids which initially smelled like renally excreted asparagus
metabolites (if you know, you know), until they were completely air-dried and odorless, and weighed 3.11 g. A melting point range of 178–191°C
(180–188°C) was determined.
[2]
The final yield of a uniformly off-white mixture of recrystallized materials seems to have been 23.58 g (69.7%).
[3] Only the much later verified melting point value exists (110–117°C). The fresh material had a very slight green tinge,
which seemed to give way to a beige hue with time. I would describe the odor as a faint, sulfury sourness, with some of the
root beer of the
ester precursor sticking through.
[1]: PiHKAL: #39 2C-T. https://isomerdesign.com/PiHKAL/read.php?id=39
[2]: At ~120°C, the material acquired a waxy appearance, as if moistened by some trace amount of
melted mercaptohydroquinone. As the sample melted, there was some simultaneous decomposition (i.e. reddening until orange), and a small amount of pale
solid material remained on the bottom. Interestingly, the previously mentioned "root beer" smell was distinctly emitted by the melted sample; this
could imply an incomplete saponification.
[3]: 19.22 g of the purified product was used and I still have 4.36 g left. This means that 2.69 g isn't accounted for in the notes,
and was most likely obtained from two initial small-scale recrystallization attempts from aqueous methanol, whose details are likewise missing — the
material should then originate from either the initial 33.19 g or [an unrecorded deduction that is missing from] the 12.8 g which was obtained from
the EtOAc recrystallization.
[Fig. 26] Rise of "pastry-like crust" under reduced pressure
 [Fig. 27] Combined fractions of crude product
[Fig. 27] Combined fractions of crude product
 [Fig. 28] Trial recrystallization from aqueous methanol
[Fig. 28] Trial recrystallization from aqueous methanol
 [Fig. 29] Combined impure fractions after seven weeks in beaker
[Fig. 29] Combined impure fractions after seven weeks in beaker
 [Fig. 30] Crystals from (the bottom of) above mixture
[Fig. 30] Crystals from (the bottom of) above mixture
 [Fig. 31] Purified material used in subsequent syntheses (surplus)
[Fig. 31] Purified material used in subsequent syntheses (surplus)

Chapter II
S-ALKYLATION
Another thoroughly fascinating feature of this approach is the exploitation of the differences between oxygen and sulfur as they relate to the atoms
being stuck on a benzene ring. A base, such as KOH, deprotonates a thiophenol in preference to a phenol due to the thiophenol being more acidic. In a
similar manner, an electrophile which is not particularly aggressive, like the alkyl bromides employed here, will have an overwhelmingly consequential
preference for a thiophenolate, which in turn is more nucleophilic than a deprotonated phenol. Therefore, what ends up happening, in this context, is
that even if one were to use more base than what is required to deprotonate the thiophenol — and even if there was a significant excess of the more
electrophilic ethyl bromide present — the immediate end result would be similar: a virtually exclusive and exhaustive alkylation of the
thiol.
[1][2]
In light of the above, it is then clear to see that using more of each alkyl bromide (and quite possibly the base as well) would more
than likely have improved the outcome of these experiments; unreacted starting material most definitely contaminated both of the products, and the
same knowledge which could have been used to largely avoid the resultant issues was used to fix them in a bit of a leap of faith, whereby bicarbonate
was used to remove the (presumed) thiophenolic impurity in open air.
Briefly, the alkyl bromides used in these reactions were prepared via dropwise addition of sulfuric acid to a cooled mixture of KBr,
water and alcohol; refluxing the mixture; distilling the product from a hot water bath; washing the product with water, aqueous bicarbonate and brine;
and drying over MgSO
4.
[1]: (Sciencemadness) Thread by Ullmann. https://www.sciencemadness.org/whisper/viewthread.php?tid=11...
[2]: (Sciencemadness) Thread by turd. https://www.sciencemadness.org/whisper/viewthread.php?tid=62...
[d4] Propylthiohydroquinone
Also called: Hydroquinonyl
n-propyl sulfide (
probably)
184.26 g/mol
Experiment 1
In a beaker, potassium hydroxide (3.13 g, 56 mmol) was dissolved in methanol (50 mL) with magnetic stirring, and the resulting rather polluted
solution
[1] was gravity filtered into a double-necked 250 mL round boiling flask through a tiny
swab of cotton wool. With the flask set up in a water bath,
[2] a dual-port gas adapter was
attached to the side neck, and used to connect both an argon bottle and a water aspirator; the vertical neck was stoppered. The alcoholic base was
then brought to a boil by pulling a vacuum which was immediately counteracted with an influx of argon to the point of (with help) lifting the stopper;
this was repeated two more times prior to replacing the stopper with a powder funnel and slowly adding 2,5-dihydroxythiophenol (7.11 g, 50 mmol)
through the sustained argon outflow. The mixture was allowed a moment of idle stirring before 1-propyl bromide (6.47 g, 53 mmol) was added in four
dropperfuls over about two minutes. Finally, the argon was terminated; the vertical neck was stoppered; and the mixture was stirred for 135 minutes.
[1]: The KOH (fig. 32) was green and contained some insoluble debris.
[2]: Both S-alkylations were performed in a ≤24°C water bath due to the unknown extent of potential exotherm. Aside from
inhibiting the volatilization of the alkyl bromides somewhat, no apparent benefit was had from doing this.
[Fig. 32] Technical grade potassium hydroxide
 [Fig. 33] Methanolic solution of KOH and mercaptohydroquinone
[Fig. 33] Methanolic solution of KOH and mercaptohydroquinone
 [Fig. 34] Reaction mixture within four minutes of 1-PrBr addition
[Fig. 34] Reaction mixture within four minutes of 1-PrBr addition
 [Fig. 35] Discoloration, likely caused by lingering atmospheric oxygen
[Fig. 35] Discoloration, likely caused by lingering atmospheric oxygen
 [Fig. 36] Post-reaction mixture
[Fig. 36] Post-reaction mixture
 Work-up
Work-up
The methanol was vacuum-boiled twice as before, and acidified (under argon) by adding a gram of 33% HCl. The walls of the flask were washed down with
MeOH using a dropper,
[1] and the mixture was vacuum filtered. The filtered, slightly off-white
solids were washed using 12.7 g of MeOH and air-dried to obtain 4.67 g of (mostly) KBr which exhibited a slight, uneven darkening on air exposure. The
filtrate was distilled under reduced pressure to concentrate it down to a volume of ~40 mL before evaporating the remaining methanol under a PC fan
and transferring the residue to a water-containing beaker in order to examine its solubility (or complete lack thereof). The water was removed by
pipetting and evaporation, leaving 8.3–8.6 g of a clear, honey-colored oil.
After a fruitless attempt to obtain crystals from dissolving exactly one gram of the crude product in heptanes (10.24 g) with a bit of propan-1-ol
(1.5 g) — and subsequently converting it into a tarry mess by adding water, distilling off the organic solvents with a bunch of said water, adding
sodium bicarbonate solution to the remainder (and the distillate) to see what would happen, and stirring the mixture in the open flask to see for how
long
[2] — the remainder was allowed to remain in open air on a watch glass. Not much change was
observed after seven days, aside from some strangely isolated discoloration near the borders of the glass, and perhaps a shift to a greener hue which
wasn't apparent at the time (fig. 41).
Inspired by the result of the preceding initial bicarbonate treatment of the ethylthio derivative (see d
3), the entire quantity of material
was transferred to a beaker using 3.6 g of ethyl acetate, and 50 mL of a 5% sodium bicarbonate solution was added. The biphasic mixture was stirred
magnetically for 5 minutes and then poured into a 250 mL separatory funnel. More EtOAc was added until, after adding ~12 g with occasional swirling,
the entire organic phase had risen on top of the aqueous one. The aqueous layer was drained into a beaker, where it was further extracted by stirring
with a 10 g portion of the EtOAc prior to being separated and acidified with 3.5 g of 33% HCl. The combined organic phases were washed with 40 g of
25% NaCl, and acidified with 0.78 g of 33% HCl. The acid-treated organic layer was gravity filtered through cotton and distilled to remove the bulk of
the solvent. The concentrated solution was poured into a shallow borosilicate dish where it solidified, affording 7.32 g (79.5%) of a cream-colored,
crystalline solid with a melting point of 69–72°C (70.4–71.4°C); this had a faint, fruity odor with hints of rubber and dehydrated onion.
Nothing was extracted from the aqueous portion.
[1]: The intention was to minimize discoloration from any residual deprotonated material getting oxidized before assimilating to the
acidified portion.
[2]: There was an immediate, progressive darkening which resulted in a nearly black mixture overnight (fig. 39). I don't have an
explanation for why this happened; the baking soda was reasonably fresh and appropriately stored, and I'm quite certain that the distilled mixture had
completely cooled beforehand.
[Fig. 37] Acidic vacuum filtrate and separated solids
 [Fig. 38] Crude product (mostly) under water
[Fig. 38] Crude product (mostly) under water
 [Fig. 39] Oxidative degradation of one-gram sample <> Also distillate
[Fig. 39] Oxidative degradation of one-gram sample <> Also distillate
 [Fig. 40] Freshly isolated crude product
[Fig. 40] Freshly isolated crude product
 [Fig. 41] Crude product after six days in open air
[Fig. 41] Crude product after six days in open air
 [Fig. 42] Above material dissolved in butyl acetate
[Fig. 42] Above material dissolved in butyl acetate
 [Fig. 43] Above solution being stirred with aqueous sodium bicarbonate
[Fig. 43] Above solution being stirred with aqueous sodium bicarbonate
 [Fig. 44] Purified propylthiohydroquinone
[Fig. 44] Purified propylthiohydroquinone
 [d3] Ethylthiohydroquinone
[d3] Ethylthiohydroquinone
Also called: 2-(ethylsulfanyl)benzene-1,4-diol
170.23 g/mol
On its surface, the ethylation appears identical to the previous propylation. However, there are a couple of considerations which were not taken into
account in performing this next experiment: firstly, ethyl bromide needs comparatively little provocation to
peace out prematurely due to its
remarkably high vapor pressure; and lastly, it would then follow that continuously pumping an inert gas through the system which contains ethyl
bromide is strictly inadvisable — that is, unless there was an actual need to do so, as well as an excess of the bromide which was sufficient to
compensate. Which there wasn't.
Experiment 1
A solution of KOH (3.13 g, 56 mmol) in MeOH (50 mL) was gravity filtered into a two-necked 250 mL reaction flask, which was positioned in a cool water
bath and connected to argon and vacuum lines via a dual-port gas adapter on the angled neck. Four cycles of deoxygenation were effected in the same
manner as in d
4 prior to adding the thiophenol (7.11 g, 50 mmol), followed by EtBr (5.80 g, 53 mmol), and stoppering the vertical neck —
this time maintaining a slight, continuous efflux of argon into the aspirator reservoir while the mixture was stirred for two hours.
Work-up
To acidify the mixture, a gram of 33% HCl was added, followed by an additional portion of 10 drops.
[1] The mixture was vacuum filtered to separate 3.77 g of KBr with an air-sensitive whiteness, and the bulk of the MeOH in
the filtrate was removed under vacuum before an exhaustive evaporation to leave a dark yellow oil.
In a bid to remove any unreacted thiophenol, the crude product was treated with bicarbonate. Using an unrecorded amount of ethyl acetate, the crude
product was transferred to a beaker, where it was magnetically stirred with 50 mL of 5% aqueous NaHCO
3 for a few minutes. The separated
organic solution was washed with 20 g of 25% NaCl, and then acidified using 6 g of 5.5% HCl prior to evaporating the solvent and thus obtaining 6.32 g
of a yellow oil.
[2] No written descriptions of the odor seem to exist.
[3] The bicarbonate partition was acidified using 2.38 g of 33% HCl and extracted using EtOAc to obtain 1.46 g of a
crystalline residue which was surprisingly light in color,
[4] and practically odorless.
Eventually, after storing the product on a watch glass in open air for 19 days,
[5] it was decided
that a second bicarbonate washing be carried out. The previously bicarbonate-treated product (6.25 g) was transferred to a beaker in 5.6 g of butyl
acetate. The solution was stirred with 50 mL of aqueous 5% NaHCO
3 for 12 minutes, after which the mixture was poured into a 250 mL
separatory funnel along with 8.4 g of additional BuOAc. Unlike before, the funnel was shaken, and quite a bit of discoloration immediately took place.
The aqueous layer was drained, acidified, and later extracted to obtain no residue. The organic layer was washed with 26 g of 25% NaCl, acidified with
1.06 g of 33% HCl, gravity filtered, and dried over 0.33 g of MgSO
4. To estimate the amount of product in the solution, 0.33 g (out of 20.5
g) was placed on a watch glass, leaving ~90 mg of residue after the evaporation of volatiles; this indicated ~5.59 g of product (65.7%).
Finally, ~11.7 g of the solvent was distilled off to leave a dark, viscous, reddish-brown oil in the flask. Prior to methylation, the flask was warmed
to ~50°C and swirled around while simultaneously purging it with argon to further remove residual volatiles.
[1]: Assuming a total volume of 0.5 mL and a density of 1.16 g/mL, this would be approximately 0.58 g.
[2]: A small sample of the oil was placed on a watch glass. Cooling in the freezer produced no crystals, but hardened the oil such
that its viscosity was comparable to something like barely malleable glass. The sample acquired a reddish discoloration on standing in open air (fig.
46); over the same span of time, the main portion remained unchanged.
[3]: I vaguely recall a modest, predominantly rubber-like smell, similar to what could be picked up from propylthiohydroquinone and
later its methyl ether —
soft and sort of sweet, comparable to rubbers that are used in some worn and household items; footwear and
cleaning equipment, perhaps?
[4]: Part of my original assumption concerning the efficacy of a bicarbonate treatment was that any unreacted thiophenolate might
rapidly oxidize all the way to a hopeless tar following deprotonation. Moreover, I was quite certain that the tar would then immediately partition
into the organic solvent, tainting the hydroquinone. In a fascinating twist, the sort of dramatic oxidation that I was expecting didn't seem to take
place (unless the mixture is shaken with air in a funnel, apparently). Initially, I supposed that the presence of ethyl (and later even butyl) acetate
had a protective effect which allowed for the atmospheric isolation of 2,5-dihydroxythiophenol. However, when its melting point was finally determined
five months later; the isolated material was observed to gradually begin browning at ~120°C; partially melt into a chocolate-colored semisolid lump
at ~167°C; and then quite sharply blacken and liquefy completely at ~170°C — while the similarly stored
actual thiophenol simply assumed
a chlorine-like color as it melted at 110–117°C. That said, I imagine that the impurity could still have been impure thiophenol, at the time.
[5]: On the first (or second) day, two tiny specks of the bicarbonate-extracted solid were placed in contact with the edges of the
puddle of crude product on the watch glass, with the intention of seeing whether they'd facilitate further solid formation or something else of the
sort. After seven days, pale streaks of ultra-fine particulate appeared to be spreading out from the solids (fig. 48), and at the end of the 19-day
period, the entire puddle was observed to have become opaque, with a ring of red discoloration around the edges (fig. 49); this was what prompted the
second bicarbonate treatment.
[Fig. 45] Impurity in BuOAc (aq. NaCl in the back) <> Product in BuOAc
 [Fig. 46] Discolored sample of product
[Fig. 46] Discolored sample of product
 [Fig. 47] Bicarbonate-extracted impurity
[Fig. 47] Bicarbonate-extracted impurity
 [Fig. 48] Streaks of particulate spreading over crude product
[Fig. 48] Streaks of particulate spreading over crude product
 [Fig. 49] Above sample after ~18 days in open air
[Fig. 49] Above sample after ~18 days in open air
 [Fig. 50] Aftermath of second bicarbonate treatment
[Fig. 50] Aftermath of second bicarbonate treatment

Chapter III
PERMETHYLATION
While the process of methylation itself is nothing out of the ordinary, the importance of protecting the hydroquinones from atmospheric oxygen is
highlighted, particularly in the first experiment, where the thiophenol is only now alkylated. To that end, I figured that I would try incorporating
acetone — which was previously used in the aqueous methylation of 4-methoxyphenol (b
1) without issues — the idea being that boiling it
under vacuum would drive out most of the dissolved oxygen. It has also been suggested that the presence of acetone can significantly inhibit the
saponification of dimethyl sulfate by the aqueous alkali,
[1] which, if true (and applicable here),
would be a nice perk. Overall, the deoxygenation seemed to work well, but the yields were mediocre; this, along with the nearly identical dark brown
coloration that each of the post-reaction mixtures acquired on standing, seems to imply an incomplete conversion.
Because the territory
felt somewhat more uncharted, the initial workup in particular involves a bunch of, as Cave Johnson
from
Portal 2 might put it, "throwing science at the wall . . . to see what sticks". Out of the things that were attempted, the obvious
organic extraction and its treatment by repeated washing with aqueous alkali appeared to be the most effective, and I would consider these steps the
bare minimum in terms of isolation — kind of like I would for any of the other methoxybenzenes.
[1]: (The Hive) GC_MS: Acetone as co-solvent. https://the-hive.archive.erowid.org/forum/showflat/Cat-/Numb...
[d2] 1,4-dimethoxy-2-(methylthio)benzene
Also called: 2,5-dimethoxythioanisole
184.26 g/mol
Experiment 1
In a single-necked, 100 mL round boiling flask,
[1] acetone (10 mL) was added to a solution of NaOH
(6.40 g, 160 mmol) in water (32 mL), and the magnetically stirred mixture was very briefly boiled three consecutive times by reducing the pressure and
immediately replacing the atmosphere with argon, whose continued outflow was then utilized to purge the subsequent addition of mercaptohydroquinone
(5.00 g, 35 mmol). The resultant solution was stirred for a couple of minutes, and a 250 mL separating funnel containing Me
2SO
4
(13.3 mL, 140 mmol) was attached, with simultaneous disconnection of the argon line to leave the apparatus open. The addition of DMS, initiated with a
rate of about 10 drops per minute, was completed in exactly one hour, with the reaction temperature hovering right around 37.4°C (per IR). The flask
was stoppered, and stirring was resumed for two hours before allowing the mixture to stand for about 52 hours at ambient outside temperatures
(13–23°C), pending workup.
[1]: The reaction flask was fitted with a vertical vacuum distillation adapter, which was connected to an argon bottle via its gas
port. A stopcocked 180° gas adapter was connected to a water aspirator, and attached to the female joint on the distillation adapter; once the vacuum
pump had served its function, the gas adapter was removed, and the starting material was added through the distillation adapter. This configuration
was utilized in all three of the methylations.
[Fig. 51] Reaction mixture prior to addition of DMS
 [Fig. 52] Reaction mixture after adding most DMS
[Fig. 52] Reaction mixture after adding most DMS
 Work-up
Work-up
The post-reaction mixture was poured into a 250 mL separatory funnel, diluted with 50 mL of water, and extracted using three portions of DCM (20, 5,
then 5 mL). The extracts were pooled and stripped of most solvent via distillation in a hot water bath, leaving approximately 5.8 g of a vibrant,
reddish-brown oil that had a fairly repulsive odor, reminiscent of dimethyl sulfide and sewage.
The usefulness of steam distillation was gauged by adding 32.67 grams of water to the crude oil and collecting ~10 mL of a biphasic distillate whose
bottom organic layer (<0.5 g) was pipetted onto a watch glass and evaporated along with two tiny portions of DCM used to crudely extract the
aqueous layer (for not much apparent gain). After a day of standing in open (24°C) air, the residue had shed most of its unpleasant cabbage-like
smell, and weighed ~90 mg. When no solidification was observed in over a week, the residue was placed in the freezer where it crystallized as a
pale-yellow mass which appeared to melt upon reaching ~18°C on standing at room temperature (lit. 33-34°C
[1] ).
NaOH (0.98 g) was added to the remaining ~25 mL of crude product and water, and the mixture was stirred for 15 minutes or so prior to extraction with
three small portions of DCM; some color remained in the aqueous alkali. 20 mL of heptane was then added to the combined extracts in a 50 mL flask, and
the mixture was distilled to remove DCM, causing some dark, solid impurity to precipitate. Another 20 mL portion of heptane was added, and
distillation was resumed until ~15 mL of the mixture remained. This was then gravity filtered through cotton, rinsing the filter twice with fresh
heptane. A heavy, red oil separated as the mixture cooled, but was retained due to it potentially containing dissolved product; an addition of 10 mL
of toluene merged everything into a clear, amber solution.
0.57 g of finely ground activated charcoal was suspended in the solution at room temperature, and the mixture was then boiled for five minutes, cooled
in a water bath, and stirred for two more hours at room temperature. The charcoal was removed by vacuum filtration through qualitative filter paper,
affording a solution which was clearly less colored, but not dramatically so. A ~1% fraction of the solution was evaporated on a watch glass to obtain
~50 mg of an oil which crystallized in the freezer, but would melt at a significantly lower temperature (>0°C) than the previously steam distilled
sample.
Three more alkaline washes were performed using 10-gram portions of 10% NaOH; the first two washes assumed shades of yellow, and the third portion
remained colorless. A total of 3 g of methanol was then added to the third washing, until it caused some more of the discoloration to partition into
the aqueous layer.
[2] The bright-orange organic solution was further shaken with a small portion
of water containing 0.52 g of 33% HCl, followed by an 11 mL portion of water with 0.51 g of dissolved potassium metabisulfite (both of which came out
colorless), before evaporation on a watch glass. The residual oil contained some trapped moisture, dust, and a fruit fly, which were separated: the
product wasn't soluble in plain heptane, but dissolved in a mixture containing 10% of toluene; this was then filtered through a ball of cotton.
Evaporation to a constant weight afforded 3.99 g of an amber oil with a faint, mildly unpleasant smell (4.08 g in total; ≤69.1%).
[1]: PiHKAL: #39 2C-T.
https://isomerdesign.com/PiHKAL/read.php?id=39
[2]: This methanol-spiked washing was evaporated; the yellow, hygroscopic residue didn't seem to contain too much of the desired
methoxybenzene, and was discarded. All in all, the incorporation of methanol was not worthwhile.
[Fig. 53] DCM extract <> Aqueous partition
 [Fig. 54] Removal of DCM by distillation
[Fig. 54] Removal of DCM by distillation
 [Fig. 55] Activated charcoal <> Solution to be treated
[Fig. 55] Activated charcoal <> Solution to be treated
 [Fig. 56] Alternative perspective
[Fig. 56] Alternative perspective
 [Fig. 57] Charcoal-treated solution of product
[Fig. 57] Charcoal-treated solution of product
 [Fig. 58] Evaporated and refrigerated sample from above solution
[Fig. 58] Evaporated and refrigerated sample from above solution
 [Fig. 59] Solution of product over dilute HCl
[Fig. 59] Solution of product over dilute HCl
 [Fig. 60] Purified product with residual moisture and airborne impurities
[Fig. 60] Purified product with residual moisture and airborne impurities
 [e4] 1,4-dimethoxy-2-(propylthio)benzene
[e4] 1,4-dimethoxy-2-(propylthio)benzene
Also called: 1,4-dimethoxy-2-(propylsulfanyl)benzene
212.31 g/mol
Experiment 1
In a 100 mL reaction flask, acetone (16 mL) was added to a stirred solution of NaOH (4.84 g, 121 mmol) in water (35 mL), and — in the same manner as
before (d
2) — the mixture was deoxygenated prior to adding 1,4-dihydroxy-2-(propylthio)benzene (7.00 g, 38 mmol) and setting up a
dropwise addition of dimethyl sulfate (10.3 mL, 109 mmol). The entire addition took 30 minutes, with the reaction temperature remaining at ~36°C (IR)
throughout. The mixture was stirred for some time following the addition,
[1] after which the flask
was stoppered and allowed to stand at ambient outside temperatures (above and below 20°C) for six days.
[1]: The exact time wasn't recorded, but most likely all three of the methylation experiments were stirred for two hours following
the end of addition.
[Fig. 61] Reaction mixture prior to addition of DMS
 [Fig. 62] Reaction mixture after adding most DMS
[Fig. 62] Reaction mixture after adding most DMS
 Work-up
Work-up
The mixture was diluted with 50 mL of water and extracted with four portions of DCM (20, 5, 5, and 5 mL). The bulk of the solvent was removed by
distillation, and the remaining oil was dissolved in 10 mL of heptanes. The addition of a 10 mL portion of 10% aqueous NaOH generated a small quantity
of dark sediment on stirring; the mixture was gravity filtered into a separatory funnel through cotton, and chased with a 10 mL portion of additional
heptane. The organic phase was retained, and washed with three more identical portions of alkali, followed by 10 mL of water, before it was drained
into a 50 mL flask. The separatory funnel was rinsed with a 10 mL portion of toluene which was also added to the flask. This nonpolar mixture was then
distilled atmospherically to the point of more dark sediment appearing, after which the distillation was resumed under vacuum until the precipitate
eventually redissolved in the residual oil. 10 mL of fresh heptane was then added, which once again precipitated the supposed impurity. The suspension
was refluxed for ~10 minutes with a gram of freshly ground activated charcoal. Vacuum filtration (whereby the glassware and separated charcoal were
rinsed with 2 mL of heptane) and evaporation of the filtrate to a constant weight gave 5.90 grams (≤73.2%) of a honey-colored oil with a mellow,
curiously zingy odor of sulfury rubber paired with some ambiguous middle ground of yellow onion and garlic.
Two weeks later, a ~0.80 g portion which wasn't used in the formylations was steam distilled. A coarse extraction of the collected slightly discolored
oil (from 200–250 mL of distillate) using DCM afforded, on evaporation, 0.69 g of an off-white, crystalline solid melting at 31.1–32.9°C
(32.1–33.7°C). The odor was unchanged.
[Fig. 63] Dark impurity resulting from treatment with base
 [Fig. 64] Steam distillation of unused product
[Fig. 64] Steam distillation of unused product
 [Fig. 65] Solid 1,4-dimethoxy-2-(propylthio)benzene
[Fig. 65] Solid 1,4-dimethoxy-2-(propylthio)benzene
 [e3] 1,4-dimethoxy-2-(ethylthio)benzene
[e3] 1,4-dimethoxy-2-(ethylthio)benzene
Also called: 2,5-dimethoxyphenyl ethyl sulfide
198.29 g/mol
The ethyl sulfide homolog was the last to be methylated. The digital notes on my phone seem to have encountered some deconstructive variation of
pocket dialing, whereby, in between the addition of dimethyl sulfate and the recorded yield, there is a section which simply states " 6 ".
Whatever
was done was definitely very similar to the previous experiments: I recall reproducing the basic washing (also supported by fig. 68,
which I couldn't place anywhere else), and noting that the product is insoluble in heptanes; conversely, I'm convinced that I skipped the activated
charcoal treatment as well as attempting to steam distill the product.
Experiment 1
In a 100 mL reaction flask, a solution of NaOH (4.84 g, 121 mmol) in water (30 mL) was prepared. Acetone (10 mL) was then added prior to deoxygenation
of the mixture and addition of the crude 1,4-dihydroxy-2-(ethylthio)benzene (<6.25 g, <37 mmol)
[1] via the established method; similarly, the argon cylinder was disconnected as part of initiating the subsequent dropwise
addition of DMS (10 mL, 105 mmol) which took 25 minutes to complete and generated a peak temperature of ~43°C (IR). After some additional stirring,
the mixture was moved to the side where it remained — stationary and outdoors — for no more than four days.
[1]: The lowest estimate for the amount of starting material used is 5.59 grams (see d
3 for details). Prior to its
addition, the viscous, oily material was diluted with a small portion of acetone, which was also used to sparingly rinse the adapter through which
said addition was made.
[Fig. 66] Reaction mixture prior to addition of DMS
 [Fig. 67] Reaction mixture at the end of addition
[Fig. 67] Reaction mixture at the end of addition
 Work-up
Work-up
The post-reaction mixture was diluted and extracted with several portions of DCM; the extract was washed with several portions of 10% NaOH solution,
before evaporating it to constant weight on a watch glass to obtain 4.85 g of a honey-colored oil which had a tangy, sickly-sweet fruit drop aroma
with a touch of rather off-putting, sulfury sourness. Depending on the actual amount of starting material used, the yield comes out at
(≤) 66.6–74.5%.
[Fig. 68] Separation of dark impurity (supposedly on treatment with base)
 [Fig. 69] All three dimethyl ethers, as utilized (Me \ Et / Pr)
[Fig. 69] All three dimethyl ethers, as utilized (Me \ Et / Pr)

Chapter IV
FORMYLATION
In recognition of my increasing vulnerability to failure, I began the formylations by dedicating a single gram of the most abundant intermediate to
doing a pilot experiment. At this point I had run out of ethyl acetate, and switching to the decidedly more stable butyl acetate elicited the idea of
incorporating it into the hydrolysis stage under reflux; this was done, and the crude yield from the pilot experiment ended up persuading me into
trying a similar procedure on the 2,5-dimethoxyethylbenzene right away, which reinforced the wishful impression that I just might be onto something.
The rest of the organosulfur compounds were then formylated — followed by the second portion of 2,5-dimethoxytoluene — and I have to admit: I
still haven't the faintest idea as to where I stand on the issue.
Here, the yields really weren't great, which begs the logical question "Why?" as well as perhaps an emotional exclamation mark or
two. Having pondered the matter, a slew of possible explanations has come to mind — none of which I can truly dispute, and many of which could have
been taking place in tandem. Through furious speculation, I found myself in a bit of a rabbit hole which I've attempted to narrate in the hopes that
it is of more interest to the chemist than it is to the psychologist.
Firstly, there's the Duff hydrolysis conundrum, elaborated on in the
4-Me post (1/5). Hypothetically, based on a statement
by the Hyperlab user miamiechin,
[1] I might assume there to have been an initial yield of up to
~80% in each case, minus whatever percentage of impurity was present in the starting material. As an interesting side note, it initially seemed like
the crude yield of the propyl homolog from the pilot experiment could, by some metrics,
[2] be
estimated to contain as little as ~10% of impurity, which would have put the yield at roughly 84%.
Building on these arcane assumptions, and the observed discrepancy between the yields of the two propyl experiments, it would appear
that a large chunk of each product might have been lost in the bisulfite purification. There's the distinct possibility of the formation and/or
precipitation of the bisulfite adduct of each aldehyde having been significantly incomplete, which seems to be supported by the observed decelerating
but seemingly everlasting precipitation of solids. Another correlation that stands out is how, instead of the usual dropwise addition of sodium
hydroxide, a concentrated sodium carbonate solution was used to decompose the obtained adduct from all but the pilot experiment; indeed, at least one
person has claimed that the use of (potassium) carbonate instead of hydroxide leads to lowered yields.
[3] However, having tried the liquid–liquid extraction of each decomposition, the logistics for such a phenomenon seem
somewhat improbable to me, as I wouldn't expect any such loss to be soluble in the aqueous phase.
As my newest piece of conjecture, I now realize that there is one more thing which I have managed to repeatedly disregard as if it
kept curing scurvy: the acetic acid. In each experiment, 2.2–2.3 g of AcOH was used for every (supposed) millimole of starting material. Each
reaction mixture was then diluted to an arbitrary degree with water before a likewise arbitrary amount of butyl acetate was used to extract it. Beyond
this point, some interesting observations could be made: for instance, a mere 1.94 g of BuOAc per mmol (of starting material) was sufficient to
extract what amounted to a 69.6% purified yield of the methyl homolog, but subsequently washing the organic solution with aqueous bicarbonate would
then cause a significant quantity of the product to precipitate. Thus, it would appear that the amount of organically co-extracted acetic acid was
decreased via neutralization as well as partitioning to the aqueous phase, forcing the poorly BuOAc-soluble and clearly very hydrophobic aldehyde out
of solution. Similar correlations for the other two compounds aren't as conspicuous, but I believe that they're there. Mostly I'm just concerned that
I've insufficiently extracted the two seemingly less hydrophobic aldehydes from the acid-containing reaction mixture, but really the bedrock that I
keep unearthing here is that individual solubility of the [precursor/aldehyde/adduct] should be the constant main focus in deciding which variables to
adjust. At the end of this post, I've included a table that I drafted for reviewing and comparing the experimental parameters; with some tweaks, I
believe that it could be a great tool for planning and
improving future Duff experiments as well.
Finally, I need to address the subject of melting points, as something strange seems to be going on with the separately S-alkylated
derivatives. Assuming that the melting point values given in PiHKAL are indeed correct, it would appear almost as if my ethylated and propylated
products had been switched at some point. However, I'm willing to risk the existential crisis of a lifetime in declaring that there is just no way
that that could have happened. And even then, the melting points wouldn't quite match — which shouldn't be an impurity issue considering the fairly
narrow ranges of 1.2°C and 0.9°C determined for the purest respective samples. Moreover, by (re-)verifying the melting point values in December, I
have completely ruled out the possibility of a thermometer malfunction. But if Shulgin didn't mix up his values; and if I didn't mix up my products
— what could have happened? Judging by the crude melting point of the pilot experiment, which at first seems to conform but then dramatically shifts
as the material is purified, it seems like the anomalous behavior could originate from the bisulfite purification process. But then, why wouldn't
something similar happen to the methyl homolog as well? It seems worth bringing up that Shulgin apparently used the Rieche (dichloromethyl methyl
ether/Lewis acid) to formylate the methylthio compound, and purified it via the bisulfite adduct;
[4] while the ethyl and propyl derivatives were formed through the Vilsmeier, and simply recrystallized from methanol until
satisfactory
[5][6] — with the latter apparently producing a perfect NMR spectrum. The best I
could do is some last-minute TLC, but characterization of any products from subsequent syntheses should in time supplement this nicely.
[1]: (Hyperlab) miamiechin: 70–80% yield. https://hyperlab.info/inv/index.php?s=&act=ST&f=17&a...
[2]: SRS - Rule-of-thumb: 1% of foreign substance will result in a 0.5°C depression. https://www.thinksrs.com/downloads/pdfs/applicationnotes/MPP...
[3]: (Hyperlab) ramboTT_1: [7] (KOH vs. K2CO3). https://hyperlab.info/inv/index.php?s=&act=ST&f=17&a...
[4]: PiHKAL: #39 2C-T. https://isomerdesign.com/PiHKAL/read.php?id=39
[5]: PiHKAL: #40 2C-T-2. https://isomerdesign.com/PiHKAL/read.php?id=40
[6]: PiHKAL: #43 2C-T-7. https://isomerdesign.com/PiHKAL/read.php?id=43
[f2] 2,5-dimethoxy-4-(propylthio)benzaldehyde
240.32 g/mol
360.49 g/mol (bisulfite adduct; potassium salt)
Experiment 1
In a 25 mL reaction flask, 1,4-dimethoxy-2-(propylthio)benzene (1.00 g, ≤4.7 mmol) and urotropine (1.32 g, 9.4 mmol) were mostly dissolved in
magnetically stirred acetic acid (6.20 g). To the mostly clear solution was then added a solution of 98% sulfuric acid (1.88 g, 18.8 mmol) in acetic
acid (4.12 g); the addition was performed dropwise, over 19 minutes, using a Pasteur pipette; and the ensuing cream-colored suspension was stirred for
five more minutes at room temperature. The flask was then fitted with a condenser, and its contents were brought up to a boil in a 1000 mL heating
mantle. After refluxing the mixture for 120 minutes, there was added butyl acetate (4.37 g) which separated a dense, dark oil. Once the heterogenous
mixture had been refluxed for another 80 minutes, water (5.03 g) was carefully added, and the heating was kept up for 60 more minutes before allowing
the mixture to cool to room temperature, at which it was then stirred for 13 hours.
[Fig. 70] Solution of substituted benzene and HMTA in AcOH
 [Fig. 71] Reaction mixture following addition of sulfuric acid
[Fig. 71] Reaction mixture following addition of sulfuric acid
 [Fig. 72] Reaction mixture prior to addition of butyl acetate
[Fig. 72] Reaction mixture prior to addition of butyl acetate
 [Fig. 73] Reaction mixture following addition of butyl acetate
[Fig. 73] Reaction mixture following addition of butyl acetate
 [Fig. 74] Reaction mixture following addition of water
[Fig. 74] Reaction mixture following addition of water
 Work-up
Work-up
The bilayered mixture was poured into a 250 mL separating funnel and shaken with additional water (25 mL) and BuOAc (10 g). Once partitioned, the
aqueous phase was extracted with two or three further 10 g portions of the ester, and the organic partitions were combined for treatments with water
(10 g), 10% sodium bicarbonate (3x10 g), and finally a second portion of water (10 g). The treated organic solution was gravity filtered into a 50 mL
flask through cotton (removing a small amount of dark sediment), and distilled until ~5 g of a yellow solution remained; this was evaporated to a
constant weight on a watch glass, yielding 1.06 g (<93.8%) of a waxy, yellow residue with a melting point of 71–77°C (lit. 76–77°C
[1] ) and a small amount of (likely inorganic) impurity which didn't melt. Dissolution of the material in
11.8 g of methanol was incomplete, even with a gram of butyl acetate as a co-solvent, but the residual solids dissolved in the plain ester (1.88 g).
A solution of potassium metabisulfite (7.53 g) in water (16.8 g) was prepared. The methanolic solution of crude aldehyde was funneled into the stirred
bisulfite through cotton wool, and the mixture promptly thickened with solids that were pale apart from some dark orange bits here and there. The
previous 1.88 g portion of BuOAc was then added, which appeared to dissolve the colored impurity. After about 30 minutes, the mixture was vacuum
filtered, and the moist filter cake was strenuously rinsed with 8 g of fresh BuOAc before being placed on a watch glass to dry. The filtered mixture
continued to develop solids; a second filtration was performed after about an hour, followed by a third one on the next day. The filtrate was retained
for further observation.
In a beaker, the accumulated (partially dried) adduct was suspended in 50 mL of water with good stirring, and 2.35 g of a 20% NaOH solution was added
to raise the pH to 10–11, followed by 5 g of BuOAc used to dissolve the solid phase. The mixture was then transferred to a separating funnel, using
an additional 2.5 g of BuOAc to rinse the beaker. The phases were separated, and a further 0.44 g of the 20% NaOH was added to the aqueous
portion
[2] prior to extracting it with two more five-gram portions of BuOAc. Once combined, the
organic extracts were washed with 10 g of 25% NaCl and a few grams of water. Most of the solvent was then distilled off, and as residual water was
removed with it, a small amount of supposed salt precipitated; the residual organic solution was drawn into a pipette through a swab of cotton and
deposited onto a watch glass to evaporate. Consequently, there was obtained 0.75 g (66.3%) of an odorless, slightly yellow, crystalline residue with a
melting point of 82.9–84.6°C.
[1]: PiHKAL: #43 2C-T-7. https://isomerdesign.com/PiHKAL/read.php?id=43
[2]: The presence of butyl acetate seemed to either buffer the pH down or distort the reaction
between the mixture and universal pH paper; a pH of ~9 was indicated, hence the addition.
[Fig. 75] Initial organic extract <> aqueous partition
 [Fig. 76] Residue from evaporation of above extract
[Fig. 76] Residue from evaporation of above extract
 [Fig. 77] Alternative perspective
[Fig. 77] Alternative perspective
 [Fig. 78] Formation of bisulfite adduct
[Fig. 78] Formation of bisulfite adduct
 [Fig. 79] Wet bisulfite adduct
[Fig. 79] Wet bisulfite adduct
 [Fig. 80] Bisulfite-purified product
[Fig. 80] Bisulfite-purified product
 [Fig. 81] Additional precipitation from retained bisulfite mixture
[Fig. 81] Additional precipitation from retained bisulfite mixture
 Experiment 2
Experiment 2
To a well-stirred solution of the benzene (4.04 g, ≤19 mmol) and HMTA (5.36 g, 38.1 mmol) in AcOH (25.55 g) in a 100 mL reaction flask, 98% sulfuric
acid (7.63 g, 76.2 mmol) in AcOH (18.13 g) was added dropwise (from a 250 mL addition funnel) over 22 minutes, causing the temperature of the mixture
to plateau at 31–32°C. Following completion, the thick suspension was stirred for seven more minutes at room temperature prior to being brought to
a reflux for 130 minutes. This was followed by one hour of stirring sans heating, after which there was added water (20.30 g) and BuOAc (14.68 g).
Stirring as such was continued for ~30 minutes, and the mixture was refluxed for an additional 40 minutes before allowing it to cool once more.
[Fig. 82] Reaction mixture being heated
 [Fig. 83] Above mixture 3 minutes later
[Fig. 83] Above mixture 3 minutes later
 Work-up (2)
Work-up (2)
After some 20 hours at room temperature,
[1] the mixture was diluted with 15 g of water, and
partitioned. The aqueous portion was extracted twice with BuOAc (12, then 21 g), and the extracts were merged with the organic portion. This was
shaken twice with 25 g portions of water in a separatory funnel and then drained into a stirred suspension of NaHCO
3 (10 g) in 90 mL of
water; an additional three grams of NaHCO
3 was required to retain alkalinity in the aqueous phase (one gram followed by four half-gram
portions). The organic solution was separated and dried over 1.02 g of MgSO
4.
Omitting distillation
[2] to remove excess solvent, a solution of potassium metabisulfite (35.52 g)
in water (78.63 g) was added to the dried organic solution with strong stirring. Solids began forming after about 10 minutes. After about 12 hours,
the mixture was vacuum filtered. The filter cake was rinsed with methanol,
[3] which appeared to
give rise to further precipitation on mixing with the filtrate.
[4] The cake was air-dried to a
constant weight of 3.44 g, and a second filtration of the mixture ~18 hours later gave 0.38 g of additional solids for a total of 3.82 g.
The twice harvested bisulfite mixture was partitioned. The organic portion was used to extract the remainder of the reaction mixture (where the two 25
g water washings had also been deposited). Another extraction was done using BuOAc (10 g), and the combined organic extracts were washed with water
(10 g), 25% NaCl (10 g), 10% NaHCO
3 (8x10 g), and 25% NaCl (10 g) again, before being dried over 1.25 g of MgSO
4 for ~20
minutes. Meanwhile, the aqueous bisulfite solution from before was replenished via dissolution of an additional 1.18 g of
K
2S
2O
5, and then combined with this new organic extract. The mixture was stirred for ~12 hours and filtered. The
solids were rinsed with MeOH (2 mL) and BuOAc (6 g). The air-dried solids (3.03 g) were merged with the previously obtained 3.82 grams.
In an attempt to ensure that all of the available benzaldehyde would be retained, the spent bilayered bisulfite mixture was combined with that of the
previous experiment. After 24 hours of standing, there had formed a bunch of additional solid material, which was separated by vacuum filtration and
rinsed with 2 mL of MeOH. This, in combination with a later 0.68 g portion of even more solid material (rinsed with BuOAc), weighed 3.85 g.
The two portions of ostensible adduct were processed forth separately, beginning with the latter. The solid was added to a ~26.2% solution of
Na
2CO
3 (17.49 g) in water (49.38 g), and the mixture was stirred for 15 minutes. There was then added 5 mL of butyl acetate,
which was noted to dissolve the solid phase sluggishly (in 10–20 seconds). The mixture was left to stand for a little over an hour before the phases
were separated and the aqueous portion was extracted with two 5 mL portions of BuOAc. The organic extracts were combined, dried over 0.31 g of
MgSO
4 and evaporated on a watch glass to yield 0.32 g of a crystalline residue with a melting point of 83.6–84.6°C.
The former 6.85 g portion was similarly decomposed in 80 g of the ~26.2% Na
2CO
3. The solid phase didn't seem to dissolve too
well, with most of it persisting after [probably 5–15 minutes] of being suspended in a stirred emulsion containing 10 mL of butyl acetate. Instead
of adding more BuOAc right away, the mixture was vacuum filtered. The filtered solids dissolved partially when rinsed with a 10 mL portion of BuOAc,
so an approach was chosen whereby the same biphasic mixture was repeatedly filtered through the solids into a separatory funnel, with BuOAc being
added incrementally until, after adding three more portions (10, 10 and 5 mL) for a total of 45 mL, only a few dozen crumbs remained.
[5]
The liquid mixture was partitioned, and the organic solution was dried over 0.64 g of MgSO
4 for >24 hours. No longer omitting
distillation (due to forgetting about
[2] ) the dried solution was distilled to remove the bulk of
the solvent prior to evaporation of the remainder on a watch glass to yield 2.38 g (52.0%) of a wintry, crystalline residue melting at 82.4–84.6°C.
A recrystallization of the combined product of both experiments (3.45 g) from ~19 mL of isopropanol gave 3.21 g (56.3%) of odorless, cream-colored
crystals with a melting point of 84.1–84.9°C (82.9–83.8°C).
[1]: It isn't specified whether the mixture was stirred or just stood there for ~20 hours.
[2]: Caution due to the observed heat-induced discoloration during the preceding workup of the 2,5-dimethoxy-4-ethylbenzaldehyde, as
well as a suspicious discoloration of the purified product from the pilot experiment.
[3]: The amount wasn't recorded; it was added in one portion using a 10 mL pipette, and was definitely no less than 2 mL. I seem to
recall using 6 mL but am way too uncertain to conclude thus.
[4]: This could mean an improvement to the rate and/or quantity of adduct formation, but it could also just be the precipitation of a
dissolved portion of the filter cake and/or some insolubilized undesired material. As mentioned previously, I believe that methanol generally has a
beneficial effect on the adduct formation through acting as a phase transfer catalyst (where applicable).
[5]: These crumbs were identified as mostly product by their mixed melting point. However, they were pending characterization long
enough to be excluded from the initial yield and its recrystallization, and, by extension, from the purified yield. I seem to have since misplaced the
crumbs, but I'd say there was probably no more than 100 mg (fig. 90).
[Fig. 84] Organic extract over aqueous bicarbonate suspension
 [Fig. 85] Treated extract being stirred with bisulfite solution
[Fig. 85] Treated extract being stirred with bisulfite solution
 [Fig. 86] Above mixture after 11 hours of stirring
[Fig. 86] Above mixture after 11 hours of stirring
 [Fig. 87] 3.85 g portion of adduct being added to aqueous alkali
[Fig. 87] 3.85 g portion of adduct being added to aqueous alkali
 [Fig. 88] Above mixture after 13 minutes of stirring
[Fig. 88] Above mixture after 13 minutes of stirring
 [Fig. 89] Above mixture following addition of butyl acetate
[Fig. 89] Above mixture following addition of butyl acetate
 [Fig. 90] Extracts from the two individual decompositions drying
[Fig. 90] Extracts from the two individual decompositions drying
 [Fig. 91] Undissolved crumbs of product on filter paper
[Fig. 91] Undissolved crumbs of product on filter paper
 [Fig. 92] Evaporation residue of bisulfite-purified product
[Fig. 92] Evaporation residue of bisulfite-purified product
 [f1] 2,5-dimethoxy-4-(ethylthio)benzaldehyde
[f1] 2,5-dimethoxy-4-(ethylthio)benzaldehyde
226.30 g/mol
346.47 g/mol (bisulfite adduct; potassium salt)
Experiment 1
1,4-dimethoxy-2-(ethylthio)benzene (4.60 g, ≤23.2 mmol) and HMTA (6.50 g, 46.4 mmol) were dissolved in AcOH (30.4 g) in a 100 mL reaction flask. To
the stirred solution was then added, dropwise, over 33 minutes, a solution of 98% H
2SO
4 (9.29 g, 92.7 mmol) in AcOH (22 g). On
completion, the mixture was refluxed for 90 minutes after which there was added (through the condenser) BuOAc (19.00 g), followed by a cautious
addition of water (10.40 g). After refluxing the reaction for another 70 minutes, it was allowed to cool for 20 minutes prior to adding a second
portion of water (10 mL). Stirring was then continued for ~19 hours at 24°C.
[Fig. 93] Addition of HMTA to substituted benzene in acetic acid
 [Fig. 94] Reaction mixture prior to heating
[Fig. 94] Reaction mixture prior to heating
 [Fig. 95] Reaction mixture approaching reflux
[Fig. 95] Reaction mixture approaching reflux
 [Fig. 96] Above mixture after 10 minutes
[Fig. 96] Above mixture after 10 minutes
 [Fig. 97] Mixture after 50 minutes of refluxing with water present
[Fig. 97] Mixture after 50 minutes of refluxing with water present
 [Fig. 98] Monophasic post-reaction mixture
[Fig. 98] Monophasic post-reaction mixture
 Work-up
Work-up
In a separatory funnel, the monophasic post-reaction mixture was shaken with 53 mL of water and 15 g of BuOAc, and the two layers were separated. The
aqueous portion was extracted twice using 10 g portions of BuOAc. Once combined, the organic portions were treated with 10% NaHCO
3 (4x20 g,
then 2x10 g) until a washing remained alkaline. The amber organic solution was dried overnight over 1.15g MgSO
4. A 0.17 g sample was
evaporated to dryness in a 10 mL beaker to obtain an unweighed, crystalline melting point sample with an unpleasant odor.
[1]
A bisulfite solution (38.7 g of K
2S
2O
5 in 86.2 g of H
2O) was added to the stirred, gravity filtered
organic solution in a 250 mL flask, along with 10 mL of MeOH. In ~10 minutes, the mixture had turned into an unstirrable porridge. The flask was
swirled occasionally over the next 14 hours prior to vacuum filtering the mixture, washing the filter cake with 6 mL of MeOH followed by a total of 20
mL of BuOAc, and air-drying the obtained solids to a constant weight of 6.28 g.
Because the dried adduct had a rather dirty yellow color which wouldn't wash away on the filter, it was magnetically stirred with 20 mL of methanol in
a 50 mL flask for about two hours. Filtration and air-drying of the mixture afforded 4.40 g of a significantly less discolored solid. The filtrate was
combined with the separated aqueous portion of the previously used bisulfite mixture;
[2] powerful
stirring of the mixture followed by vacuum filtration, and drying of the separated solids, gave 1.53 g of material which was likewise much less
discolored than what was had before.
A dark, crystallizing oily substance was observed to appear on top of the mixture of washings and extracted reaction mixture; a ~40 mL portion of the
distilled organic phase from the bisulfite mixture was used to re-extract the mixture. The extract was treated with two portions of 10%
NaHCO
3 (40 g, 30 g) followed by 10 g of 25% NaCl, and then stirred overnight with the pre-used aqueous bisulfite to receive 3.18 grams of
additional precipitate.
The accumulated adduct was added to 83 g of stirred ~26.2% sodium carbonate solution. After waiting 15 minutes, 10 mL of BuOAc was added, and the
mixture was stirred for 10 minutes. The solid phase dissolved partially. Two more five-milliliter portions of the ester were needed to obtain a clear
mixture; these were added 10 minutes apart. A small quantity of unidentified debris appeared at the liquid–liquid interface of the still mixture,
and was gravity filtered out. The organic phase was separated, and the aqueous phase was extracted twice more with 10 mL portions of BuOAc. The
combined organic extracts were dried over 0.76 g of MgSO
4 for ~24 hours, and concentrated through distillation under reduced pressure.
Following evaporation of the residual volatiles, there remained 2.62 g of slightly sticky, yellow crystals melting at 73.6–79.8°C (lit.
87–88°C
[3] ). These were recrystallized from ~28 mL of isopropanol, giving 2.38 g (45.3%) of
crispy, odorless crystals which were lighter in color and had a melting point of 77.1–78.8°C (76.9–78.1°C).
[1]: For whatever reason, it seems that the melting point was never determined. But there
is a picture of the sample (fig.
101).
[2]: In hindsight, it seems likely that much of the dissolved portion consisted of the adduct, and instead of just putting it back
(and probably hurting the yield in doing so), it would have been prudent to evaporate the MeOH and examine the residue.
[3]: PiHKAL: #40 2C-T-2. https://isomerdesign.com/PiHKAL/read.php?id=40
[Fig. 99] Aqueous portion of diluted reaction mixture <> Organic extract
 [Fig. 100] Washed extract <> Washings and above aqueous portion
[Fig. 100] Washed extract <> Washings and above aqueous portion
 [Fig. 101] Underutilized sample of impure product
[Fig. 101] Underutilized sample of impure product
 [Fig. 102] Treated extract being stirred with bisulfite solution
[Fig. 102] Treated extract being stirred with bisulfite solution
 [Fig. 103] Above mixture after 12 minutes of stirring
[Fig. 103] Above mixture after 12 minutes of stirring
 [Fig. 104] Dried bisulfite adduct
[Fig. 104] Dried bisulfite adduct
 [Fig. 105] Decomposition of adduct
[Fig. 105] Decomposition of adduct
 [Fig. 106] Above mixture after adding 20 mL of butyl acetate
[Fig. 106] Above mixture after adding 20 mL of butyl acetate
 [Fig. 107] Evaporation of extract from adduct decomposition
[Fig. 107] Evaporation of extract from adduct decomposition
 [Fig. 108] Alternative perspective (distorted white balance)
[Fig. 108] Alternative perspective (distorted white balance)
 [e2] 2,5-dimethoxy-4-(methylthio)benzaldehyde
[e2] 2,5-dimethoxy-4-(methylthio)benzaldehyde
212.27 g/mol
332.44 g/mol (bisulfite adduct; potassium salt)
Experiment 1
In a 100 mL reaction flask, there was prepared a solution of 2,5-dimethoxythioanisole (3.99 g, ≤21.7 mmol) and hexamine (6.08 g, 43.4 mmol) in AcOH
(29 g). A solution of 98% H
2SO
4 (8.68 g, 87.0 mmol) in AcOH (19 g) was then added — dropwise, and with strong stirring — in
30 minutes, during which the reaction mostly took place at ~31°C. Following completion, the mixture was stirred for an additional 10 minutes at
ambient 24°C prior to being refluxed for two hours. Water (10 g) and BuOAc (10 g) were then added, followed by another portion of water (10 g) after
45 more minutes of refluxing, at which point the heating was discontinued. After six hours of stirring, the mixture was warmed to 70°C (with a
short-lived intention of further refluxing) and allowed to cool again. The mixture was stirred for 17 more hours and allowed to stand for another two.
[Fig. 109] Solution of substituted benzene and HMTA in AcOH
 [Fig. 110] Reaction mixture following addition of sulfuric acid in AcOH
[Fig. 110] Reaction mixture following addition of sulfuric acid in AcOH
 [Fig. 111] Reaction mixture approaching reflux
[Fig. 111] Reaction mixture approaching reflux
 [Fig. 112] Reaction mixture after 110 minutes of reflux
[Fig. 112] Reaction mixture after 110 minutes of reflux
 [Fig. 113] Reaction mixture prior to second addition of water
[Fig. 113] Reaction mixture prior to second addition of water
 [Fig. 114] Monophasic post-reaction mixture
[Fig. 114] Monophasic post-reaction mixture
 Work-up
Work-up
The mixture was transferred to a separatory funnel where it was diluted with 50 g of water, causing a great deal of precipitation; the addition of 10
g of BuOAc redissolved the solid phase on shaking. The organic phase was collected and combined with two more ester extractions (12 g, 10 g), and the
resulting mixture was treated with two 50-gram portions of 10% NaHCO
3; this produced a pale precipitate
[1] which required several iterative solvent additions to redissolve: in order, there was added, portionwise, BuOAc (12 mL),
DCM (1, then 2 mL), water (20 mL), and DCM (7, 10 and 5 mL). Finally, only a few solid particles remained, and the biphasic mixture was partitioned.
Treatment of the product-containing partition was resumed with a further 5 g of the aqueous bicarbonate, followed by 10 g of 25% NaCl. There was then
added 0.97 g of MgSO
4.
[2]
The mixture was distilled to remove DCM, which was then used to further extract the remainder of the reaction mixture, where solids had now
formed.
[3] The latter extract was concentrated and then evaporated to yield 440 mg of a vibrant,
reddish-orange residue with a rather vomit-inducing smell, reminiscent of the truly visionary delicacy known as stockfish; melting point 71–91°C.
To the former extract, there was added a bisulfite solution (36.51 g of K
2S
2O
5 in 81.01 g of H
2O) and 10
mL of methanol; the mixture almost immediately became unstirrably thick. Vacuum filtration of the mixture was performed a total of three times, as the
filtrate would thicken once more before finally producing a ~100 mg portion of additional solid. Thus, in total, there was obtained 8.74 g of dried
precipitate.
The obtained adduct was suspended in 85 g of ~26.2% Na
2CO
3, and the mixture was stirred for 15 minutes before adding 15 mL of
BuOAc, which resulted in no discernable dissolution but rather constituted a mesmerizing, doughy vortex with the hydrophobic benzaldehyde. When an
additional 15 mL portion of BuOAc (and 10 minutes of stirring on either side of it) made no apparent difference to the quantity of solids, the mixture
was vacuum filtered through qualitative filter paper, and the filter cake was rinsed with 100 mL of water to obtain, after air-drying, 3.01 g of a
pale, greenish-yellow solid exhibiting a slightly incomplete melting at 97.6–99.6°C (lit. 99–100°C
[4] ). The organic portion of the filtrate was washed with the previous 100 mL portion of water, filtered, and dried over
0.39 g of MgSO
4; approximately two percent (0.51 g) of the solution was evaporated to obtain ~10 mg of a residue melting at 99.0–99.6°C.
Recrystallization was trialed by first gauging the solubility in 2-propanol: a 0.50 g portion of the solid material was dissolved in ~8 mL of the
alcohol, and the boiling mixture was gravity filtered through cotton to remove a small quantity of insolubles; by visually inspecting the crop of
crystals from cooling the liquor, the ratio of solvent was deemed apt. The rest of the solids were then dissolved in 32 g of 2-PrOH, gravity filtered,
and combined with the two fractions in alcohol and BuOAc. The mixture was distilled to remove alcohol
[5] and placed in the freezer. Vacuum filtration of the <0°C mixture gave 3.20 g (69.6%) of nearly colorless but
deceivingly fluorescent, odorless crystals with a melting point of 101.0–101.5°C (96.2–96.9°C).
[6]
[1]: This (along with the other instances of unexpected precipitation) was most likely the product, whose solubility in butyl acetate
seemed to be the lowest of the three homologs (in fact, of all of the benzaldehydes so far). In the notes, I haven't specified whether it was the
first or the second bicarbonate washing which caused the precipitation, and it could be that both portions ended up in the funnel at the same time
with all of the additional organic solvent, with the third five-gram portion being used to verify complete neutralization of the acid.
[2]: It is unclear if the solution was actually dried (and filtered) prior to the subsequent distillation; DCM is a considerably
better solvent for the product than BuOAc, ergo, its (sufficient) removal should precipitate the product — mixing it with the sulfate, if the
sulfate was present. In any case, it does seem like I made it to the bisulfite purification with no such incident.
[3]: Another detail was omitted in not noting whether the re-extracted mixture contained the previous bicarbonate washings; as I
recall, it did.
[4]: PiHKAL: #39 2C-T. https://isomerdesign.com/PiHKAL/read.php?id=39
[5]: The final volume of solvent was crudely estimated (after the fact) to be ~35 mL.
[6]: The verified value was obtained by placing the sample in the furnace whose temperature was already quite close to the melting
point; the abrupt heating seems to have been the cause of the slight depression.
[Fig. 115] Precipitation on rinsing reaction flask with water
 [Fig. 116] Precipitation on aqueous dilution of reaction mixture
[Fig. 116] Precipitation on aqueous dilution of reaction mixture
 [Fig. 117] Precipitation on washing extract with aqueous bicarbonate
[Fig. 117] Precipitation on washing extract with aqueous bicarbonate
 [Fig. 118] Spoonful of bisulfite adduct from the source
[Fig. 118] Spoonful of bisulfite adduct from the source
 [Fig. 119] Precipitation during secondary extraction of reaction mixture
[Fig. 119] Precipitation during secondary extraction of reaction mixture
 [Fig. 120] Secondary extract
[Fig. 120] Secondary extract
 [Fig. 121] Evaporation residue of above extract
[Fig. 121] Evaporation residue of above extract
 [Fig. 122] Dried bisulfite adduct
[Fig. 122] Dried bisulfite adduct
 [Fig. 123] Decomposition of adduct in aqueous sodium carbonate
[Fig. 123] Decomposition of adduct in aqueous sodium carbonate
 [Fig. 124] Water floating on aldehyde floating on water — under BuOAc
[Fig. 124] Water floating on aldehyde floating on water — under BuOAc
 [Fig. 125] Above mixture, vigorously stirred
[Fig. 125] Above mixture, vigorously stirred
 [Fig. 126] Solids from vacuum filtration of above mixture
[Fig. 126] Solids from vacuum filtration of above mixture
 [Fig. 127] Purified aldehyde under 2700K white LED (corrected WB)
[Fig. 127] Purified aldehyde under 2700K white LED (corrected WB)
 [Fig. 128] Purified aldehyde under UV-A
[Fig. 128] Purified aldehyde under UV-A
 [Fig. 129] Illumination: Indirect evening sunlight <> UV-A
[Fig. 129] Illumination: Indirect evening sunlight <> UV-A
 [Fig. 130] Table for reviewing experimental parameters
[Fig. 130] Table for reviewing experimental parameters
 Thin-layer chromatography
Thin-layer chromatography
To lessen the ambiguity of this post, I decided to squeeze in some TLC; despite some initial perplexity, useful results were obtained. Samples in the
approximate range of 3–6 milligrams were placed in test tubes, each containing a gram of isopropanol. Four identical plates (25 x 75 mm) were
prepared by applying a spot of each sample, which were labeled as
A (Me),
B (Et), and
C (Pr). Each
of the plates was developed using a different mobile phase before being placed in chronological order and photographed under UV-A and UV-C lighting.
Mobile phases, from left to right:
[1] Toluene
[2] Toluene/Propan-2-ol (9:1)
[3] Heptanes/Butyl acetate (10:1)
[4] Heptanes/Butyl acetate (3:1)
Retardation factors (RF), from left to right:
[1] ~0.17
[2] 0.69–0.70
[3] A: 0.15;
B: 0.19;
C: 0.27
[4] A: 0.32;
B: 0.40;
C: 0.47
[Fig. 131] Samples being dissolved in isopropanol
 [Fig. 132] Developed TLC plates under UV-A
[Fig. 132] Developed TLC plates under UV-A
 [Fig. 133] Developed TLC plates under UV-C
[Fig. 133] Developed TLC plates under UV-C
 [Fig. 134] Recrystallized benzaldehydes (Me / Et \ Pr)
[Fig. 134] Recrystallized benzaldehydes (Me / Et \ Pr)

So, here we are, with all three of the intended benzaldehydes
* in modest quantities. I'd be lying if I said I wasn't thrilled with
the result (and having gone through with sharing it to the best of my ability), but I'd also be remiss if I didn't disclose that I
am
sweating profusely from the mere thought of still needing to get to my ultimate goal of 3–6 amines with what I have. That said, there are other
targets in the 4-alkylthio realm that I'm interested in (for instance, the rather
spooky 2C-T-21), so, for better or for worse, having
another go from scratch isn't entirely unlikely. Or undesired.
* Assuming that PiHKAL contains a couple of bad melting points.
Gulp.
The final 2,4,6-trimethoxybenzaldehyde post will be somewhat more delayed, as I'll be unable to dedicate quite as much of my time to putting it
together.
But it
will come.
And it
will be nice.
[Edited on 6-1-2023 by Benignium]For your consideration:
2,4,6-trimethoxybenzaldehyde
196.20 g/mol
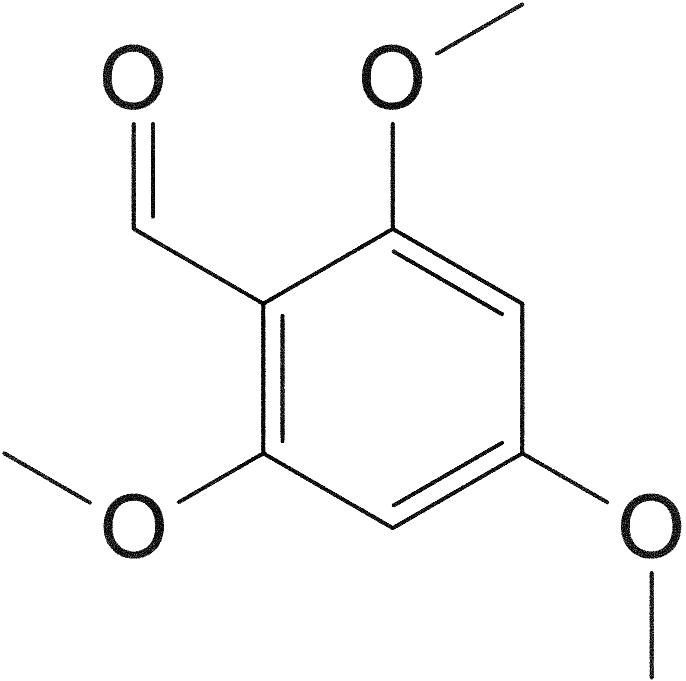
In 1884, researchers Wilhelm Will and Karl Albrecht of the Friedrich Wilhelm University in Berlin published an
article [1] in the scientific journal Berichte der Deutschen Chemischen Gesellschaft
regarding their ongoing work on assigning the appropriate trihydroxybenzene building blocks to two natural dioxycoumarin compounds, esculetin and
daphnetin. So far, daphnetin was known with certainty to be derived from pyrogallol (1,2,3-benzenetriol), but whether its constitutional isomer
esculetin conformed to the structure of hydroxyquinol (1,2,4) or phloroglucinol (1,3,5) was still up for debate. The scope of their publication,
therefore, was to identify the specific benzenetriol component of esculetin, while simultaneously producing precious experimental data on the
derivatives through which they intended to do so. In essence, three distinct triethoxybenzoic acid isomers and their ethyl esters were to be formed
from pyrogallol and phloroglucinol, [A] which could then be compared with analogous derivatives of
the unidentified benzenetriol obtained from natural esculetin. [B]
[Fig. 1] Structures of daphnetin and esculetin
In a surprising turn of events, when the carboxylic acid derivative of phloroglucinol was dissolved in ethanol and subjected to a
strong acid catalyst in an attempt to form the ethyl ester, [C] an evident decarboxylation took
place instead. Moreover, the observed liberation of gaseous carbon dioxide was accompanied by a further transformation into something which was found
to crystallize as long, white needles. This new material withstood heating in alcoholic alkali, and its reaction with ferric chloride was neither the
intense blue of the phloroglucinic acid nor the violet that was expected of phloroglucinol. Fascinatingly, it turned out that the main product of the
procedure was, in fact, the diethyl ether of phloroglucinol. [D] What's more, it was also
recognized that unlike phloroglucinol, this diethylated material could be exhaustively ethylated via the conventional Williamson ether synthesis to
obtain pure 1,3,5-triethoxybenzene, whose novel melting point would then inform the accurate conclusion that esculetin bore the structural motif of
hydroxyquinol. This pioneering work with its serendipitous discoveries garnered significant attention toward the onionesque morass that was
phloroglucinol; an issue that would vex chemists for decades to come; enduring so far as to stare back at me from the pages of PiHKAL [2] — and while the thick, proverbial jungle which once dictated these paths of quasi-alchemical
adventure and niche camaraderie may be long-macadamized by the migratory march of science, the occasional unsuspecting amateur remains likely to have
their jimmies rustled by the perpetually gaping manhole of yesteryear.
But indeed, what does it mean?
In the context of this post, it is crucial to know that attempts at electrophilic methylation of the deprotonated phloroglucinol are
prone to ending miserably: instead of the desired trimethyl ether, such a reaction would afford an overwhelming number of side products, with the
neutral portion consisting mostly of alicyclic materials like 2,2,4,4,6,-pentamethylcyclohexane-1,3,5-trione, due to rampant nuclear alkylation at the
three α-carbons. While the relative quantities of the formed side products are wildly unpredictable, the hexamethyltrione has been demonstrated as
the major (ultimate) product. By all accounts, isolating any fraction of formed 1,3,5-trimethoxybenzene from this mixture would be deemed as an
exercise in futility. [3] [4] [5] [6]
The issue was quickly likened to a ketone-like behavior by Adolf von Baeyer, who, in 1886, described the formation of the
trioxime of phloroglucinol, and proposed that the facultative intermediary be called pseudophloroglucinol; [7] the term tautomerism had just been coined by Conrad Laar, in 1885, but was initially met with a pseudo
label of its own. [8] By forming the trioxime, Baeyer had experimentally proven the existence of
cyclohexane-1,3,5-trione — the triketone tautomer of phloroglucinol — whose exact identity and implications nevertheless remained indeterminate.
At the time, experimental chemistry was in many ways constrained by its severely nascent theoretical foundation. Thus, finding new and improved ways
through this particularly loosely comprehended polyolic black box of tautomerism would continue to rely on tentative experimentation for
decades to come.
In the meantime, the early 20th century arrived, bringing with it infrastructural breakthroughs like the mass production of
affordable cars, widespread electrification, electric toasters, and sliced bread; all of which contributed to an unprecedented rate of scientific
development. In terms of chemistry, on the other hand, a revolution blossomed within the confines of the mind: Spurred, in part, by the maturation of
August Kekulé's autophagous snake anecdote into the driving force behind Johannes Thiele's rudimentary yet pioneering prediction of resonance in
1899, [9] [10] those who sought chemical understanding at the atomic level would draw it
increasingly from theoretical physics, and in doing so would align the two previously segregated fields toward an increasingly common goal. This shift
in philosophical paradigm facilitated the formulation of groundbreaking heuristic techniques like the Lewis structure, and built the foundations of
quantum chemistry. Then, finally—through the concordant invention of modern experimental tools like the concept of pH and NMR spectroscopy, that
could supersede traditional staples like elemental analysis and spruce chips wetted with acid and alcohol [11]—the stage was set for demystifying phloroglucinol once and for all.
In 1956, by comparing the ultraviolet absorption spectra of increasingly alkaline phloroglucinol solutions with those obtained
previously for 1,3,5-benzenetriamine at various stages of protonation, Hans Köhler and Günther Scheibe discovered correlations which led them to
deduce the existence of analogous isosbestic points, and, notably, to predict the large prevalence of a ketonic dianion form through comparison with
the dianion of filicinic acid, whose structure was known. [12] Corroborating the same phenomena,
studies making use of 1H NMR in the 60s would then independently reveal ring protonation to form σ-complexes (i.e.,
arenium ions) in both superacidic [13] and alkaline [14] media. More recently, in a 1993 report by Martin Lohrie and Wilhelm Knoche, UV-visible and diode-array
spectrophotometers were augmented with stopped-flow apparatus and temperature jump techniques in order to observe the chemical kinetics and
thermodynamics of altering the pH in aqueous solutions of phloroglucinol; thereby arriving at a wonderfully comprehensive view of the different
equilibria pertaining to its dissociation and tautomerism (fig. 2). [15]
[Fig. 2] Illustrated species of phloroglucinol
The above Scheme II illustrates most of the structurally distinct species of phloroglucinol. Each
horizontal row depicts the different degrees of dissociation (i.e., of protonation/deprotonation) within a single degree of conjugation; with
each vertical column therefore representing the range of conjugation within a single degree of dissociation; and with structures A
and B being intermediates for acid-catalyzed reactions of 1 to 7 and 7 to
9. On adjusting the pH in either direction, two distinct kinds of reaction were observed to take place: the horizontal double arrows
indicate diffusion-controlled (unmeasurably fast) acid–base interactions that occur independently of tautomerism, whereas diagonal arrows denote the
associated rate-determining keto–enol rearrangements. The embedded Figure 9 presents the species' relative concentrations
on a logarithmic scale as functions of the pH.
In the following paragraphs, I've attempted to explain the phloroglucinol issue in a way that might have enabled myself to more
rapidly grasp the basic concept some ten months ago; for this, I'll continue referring to structures from the above illustration as corresponding
digits in bold.
From the perspective of organic synthesis — specifically, of electrophilic O-alkylation in polar, protic media — in order for an
electrophile (like the dimethyl sulfate whose application is described in this post) to get attacked by the nucleophilic oxygens of phloroglucinol,
each phenol needs to be activated through basic deprotonation; much like has been done to the previous phenols of this thread. This removal
of what is essentially the bare nucleus of a hydrogen (and a major building block in heavier nuclei) results in the negatively charged phenolate
(i.e. phenoxide) anion—the conjugate base of phenol (i.e., a better nucleophile than phenol)—enriched by a third pair of
unbonded valence electrons, which, albeit delocalized due to being conjugated with the aromatic system (i.e., occupying a p-orbital on an
oxygen that is coplanar and in phase with an adjacent benzene carbon), remains subject to the inductive attraction which oxygen exhibits owing to the
higher number of the positively charged protons contained in its nucleus compared to the larger carbon (i.e., its higher electronegativity).
This added charge density seems particularly significant in the phloroglucinol structure, as the three ortho- and para- (but not
meta-)activating phenols are all located meta to each other, leaving the three α-carbons in their midst unusually electron-rich
through π donation such that C-alkylation has been shown to occur and even predominate in the absence of base; when the neutral molecule exists
almost exclusively in its aromatic form, 1. [16] The phenolate monoanion
2 (and by extension the appropriate monoether anion), in contrast, has been successfully reacted with dimethyl sulfate in the
presence of carefully controlled quantities of alkali to produce the O-permethylated ether. [3] [17] [E]
As far as the dianion is concerned, however, it is by now evident that the prediction of a prevailing dibasic phenolate form
(3) is turned on its head by the phenomenon referred to as keto–enol tautomerism—the proton-transfer equilibrium enabling
transformations between a nucleophilic enolic form (an alkene situated adjacent to an alcohol) and an electrophilic
ketonic form (a carbonyl with an accommodating α-carbon), catalyzed by both donors and acceptors of protons (i.e., acids and bases in the
Brønsted sense, respectively). Judging by the previous observations of predominant C-alkylation, it follows, therefore, that the prevalence of the
ketone-like character following a second deprotonation is high enough to almost completely exclude the oxygen atoms from interacting with the
alkylating agent — while the α-carbons move up in pecking order, collectively consuming (in total) up to six molar equivalents of the
same.
At a glance, the aromaticity that is ubiquitous in phenols might be expected to favor a desirable polyenolic arrangement — after
all, the equivalent of this is known to be the case even for the symmetric benzenediol, hydroquinone, whose stability is accordingly greatest when
there is a continuous ring of conjugated p-orbitals in a planar conformation (i.e., aromaticity) — However, rather than going from
1 → 2 → 3 → 4 in a straightforward series of deprotonations, as the already unusual electron density of phloroglucinol is made
even greater, it begins rearranging in exquisite ways to compensate, promptly doing away with its aromatic character; the urgency with which these
rearrangements take place is equally striking, and likewise reflected by the overlap between the first two of its acid dissociation constants,
pKa1 = 8.6–9.0 and pKa2 = 8.9–9.1 (with pKa3 = 14). [15] Nevertheless, even when staring at the above Scheme II, the reasoning behind this observed
inclination is not immediately obvious.
In mulling the subject over, guidance was sought, and two hints were received which were found specially fruitful.
The first one points to structures with two carbonyls sharing an α-carbon: A well-established quirk of nature, these are commonly
referred to as 1,3-dicarbonyls or (where applicable) β-diketones, and are almost universally introduced at a point during university lectures on
organic chemistry in the form of a β-keto ester product of a Claisen condensation. Belonging under a larger phenomenological classification called
active methylene compounds [where there’s a methylene in between two electron-withdrawing groups (e.g. -NO2, -CN, -COOR)], their
distinctive feature is an sp3-hybridized carbon center with particularly labile (i.e. acidic) hydrogens, whose dissociation gives
a highly resonance-stabilized conjugate base; communicated via a general approximation of pKa = 9. Indeed, implicit postulates of such
a diketonic anion are many for phloroglucinol (as well as resorcinol and other similarly related substances), and the structures of Scheme
II approach it as closely as limiting the portrayals of negative charge to the oxygens seems to allow.
The other concept deserving of a good grasp has to do with the stability of a structure as it relates to bond (dissociation)
energies. On its own, this value, determined for a given bond at its equilibrium length, measures the amount of energy that would be required to
completely negate the interactive forces between two covalently bonded atoms by pulling them apart. Consequently, the sum of these individual values
contained within a given molecule can be calculated to evaluate the stability and thermodynamic favorability of said molecule. The total energies were
calculated for the kinetic dianion (3) and the proposed thermodynamic dianion structure according to values obtained from a single
literature source. [18] As such, these came out to be 5590–5804 kJ/mol and 5738–5959 kJ/mol,
respectively, which would tip the scales in favor of the diketone tautomer. However, after correcting the former value through incrementing it by 151
kJ/mol according to the aromatic stabilization observed for benzene, the two values appear to converge, obfuscating whatever evidence is
there.
It is my belief that the combined bond energy of the proposed thermodynamic product is complemented by a superior distribution of
charge between a single enolate anion and the 1,3-dicarbonyl anion whose smear of electron probability (another cool way to think about
electron density) quite exactly opposes the more localized charge of the former, reducing the overall dipole moment of the structure. Below is a
somewhat streamlined illustration of the logistics for arriving at the discussed dianion structure as I currently understand them; referring to
Scheme II, this progression would correspond to 1 → 2 → 3 → 6, at which point it splits into two
distinct routes via 5, either through 8 or by way of resonance. Alkylation (particularly involving the smaller, less
sterically hindering alkyl groups) of the dissociated active methylene in between the carbonylic oxygens will serve to further stabilize the charge
toward the substituted α-carbon through σ donation by the alkyl substituent—facilitating geminal alkylation prior to a structural or
canonical (i.e., mesomeric, as in "resonance contributor") shift to favor another one of the ring carbons in the same way. The
six-membered ring structure of the dianion favors alkylation at the carbon by constraining the 1,3-dicarbonyl anion into a W-shaped conformation,
where the nucleophilic middle protrudes, and the carbonylic oxygens are pointed away such that they do not hinder its access or affinity toward
electrophilic substrates; moreover, this conformation minimizes the dipole-dipole repulsion at the site, which contributes to stability.
[Fig. 3] Favorable pathways to the thermodynamic dianion structure
But wait, there’s more!
What has been discussed on the theory so far leaves out a bunch of nuance which I’d like to touch on briefly before a few more
words on the practical side of things.
As previously stated, the above focuses on changes that take place in prot(on)ic (i.e. hydrogen-bonding) solvents like water
and alcohol. These can interact with the anionic species of phloroglucinol and its alkylated derivatives, as well as the cation and the alkylating
agent. Interaction like this can significantly favor C-alkylation by solvating (i.e. surrounding) and thus effectively shielding the oxygen
of an enolate anion; similarly, it can also hinder the desired reactivity by encumbering the alkylating agent. Additionally, a protic solvent can act
as an acid or a base in tautomeric proton-shifts. The use of polar solvents in this context is a given, since a heterogenous reaction with an
undissolved solid salt (whose oxygen anion is shielded by the associated cation in the crystal lattice) would vastly favor C-alkylation. The use of
aprotic solvents—and especially dipolar aprotic ones [i.e., ones with a sizable dipole moment (e.g. DMF, DMSO,
MeCN)]—on the other hand, tends to favor O-alkylation through not only the lack of hydrogen-bonding, but also a proportionally stronger solvation of
the metal cation relative to the enolate anion, which is consequently freer to attack with its more electronegative atom. [18] [19]
Furthermore, the concept of hard–soft character (i.e. polarizability) in Lewis acids and bases has been found to correlate
with a superior selectivity for O-alkylation exhibited (in some solvents) by dialkyl sulfates, whose hard sulfate leaving group is more amenable to
coordinating (i.e. bonding) to the hard oxygen donor atom of an ambivalent metal enolate in comparison to a softer vicinal carbon donor.
Conversely, the soft character of alkyl halides (which decreases with the atomic radius of the halogen) explains why their involvement favors
C-alkylation. It is also worth noting that the carbon under nucleophilic attack in an SN2 transition state favors attack by the less
electronegative site of an ambident nucleophile. Among the hard Lewis acids that are most often associated with a phenolate anion, the size of the ion
has a significant impact on selectivity, whereby increased bulk (e.g., K+ > Na+ > Li+) corresponds to
an increased tendency to dissociate, and therefore a higher reactivity of the anion as a nucleophile. [18] [19]
In the gas phase, where the enolate anion (of cyclohexanone) is completely free, there is only O-alkylation. [18]
A striking irony of the phloroglucinol subject is that so much of this newfound understanding on it from the past century seems to
have had no bearing on the seldom undertaken laboratory preparation of its trialkyl ethers. The most commonly encountered procedure (among the amateur
community, at least) still seems to be the same one that is featured in PiHKAL (which itself is only a slight variation of the 1884 procedure by Will
and Albrecht), where phloroglucinol is reacted with an excess of alcohol in the presence of a strong acid catalyst; [F] followed by a bimolecular nucleophilic displacement (SN2) reaction of the resulting dialkoxyphenol with an
electrophilic alkylating agent in the presence of a base (i.e., the Williamson ether synthesis). Nevertheless, interesting experimental
discoveries have been made. In closing, here are some of the highlights:
As early as 1900, F. Kaufler suggested that the deprotonated phloroglucinol acts as a ketone toward methylation, but as a phenol
toward benzylation; [20] a seemingly reasonable observation of steric effects, whose preciseness
has since been disputed. [21]
In 1920, K. Freudenberg found that reacting the phloroglucinol diethyl ether with 5.32 molar equivalents of dimethyl sulfate (based
on phloroglucinol) gave improved yields of up to 80%. [22] [G]
A 1953 publication by H. Bredereck, I. Hennig and W. Rau reports success in circumventing the C-alkylation-favoring tautomeric forms
by employing a dropwise addition of 20% NaOH to a mixture containing 3.86 molar equivalents of dimethyl sulfate and 1.9 mL of acetone per gram of the
anhydrous phloroglucinol while maintaining a constant pH in the range of 8–9; colorless crystals corresponding to a 93% yield were reported [17] [H] — in contrast, a dropwise addition of alcoholic base had previously been trialed by J. Herzig
and F. Wenzel in 1906, with modest yields and some C-alkylation being reported. [3]
Arguably the most convenient-sounding procedure for direct trialkylation was presented by J. W. Clark-Lewis in 1957, involving the
portionwise addition of DMS to a mixture of anhydrous phloroglucinol and potassium carbonate in acetone. [23]
The esterification using acetic anhydride proceeds in a straightforward manner to yield the 1,3,5-triacetoxybenzene, which has been
subjected to alkylation in the presence of base so that the ethers form just as the electron-withdrawing acetates are saponified, keeping the electron
density low enough to avoid C-alkylation. [6]
Finally, I would like to state my heartfelt gratitude for my fellow SM member AvBaeyer, whose
compassionate gift of awe-inspiring expertise was pivotal for my learning and composing of the above.
Notes
[A]: The carboxylic acid moiety was introduced, in yields that were reported as nearly quantitative, by digesting the
benzenetriols [in water, at 130°C, in a pressure reactor] with four equivalents of ammonium carbonate (or in the case of phloroglucinol, with
potassium carbonate).
[B]: In a later publication, Will outlines the processing of naturally derived glycosidic materials, including
esculetin, as follows: A water-soluble cleavage product, usually a phenolic ester, is first saponified using potassium hydroxide, followed by
treatment with methyl (or ethyl) iodide at reflux. The resultant ether is oxidized with potassium permanganate to remove the unwanted, mostly
unsaturated aliphatic ring substituents. A dry distillation of the calcium carboxylate derivative is then employed to isolate the corresponding phenol
ether, whose constitution (once further purified via steam distillation, in the case of esculetin) can be determined by comparison with the
synthetically prepared phenol ethers. Additionally, this article reports the purification of 1,3,5-trimethoxybenzene via steam distillation after
adding some KOH solution. [24]
[C]: Interestingly, although the process is very similar to the Fischer–Speier esterification described in 1895 (also
in Berlin), it is credited to Hugo Schiff, who in turn refers to the preparation of ethyl gallate by the French chemist Édouard Grimaux in
1864.
[D]: More specifically, a mixture of products was determined which contained some 10% of the monoether. [25]
[E]: Since the monoanion of phloroglucinol has been shown to not predominate at any pH, [15] I suspect that the successes of these procedures may be largely due to its diffusion-controlled formation and subsequent
ability to react immediately in the presence of excess electrophile.
[F]: The formation of phloroglucinol mono- and dialkyl ethers in Fischer–Speier-like conditions is thought to occur
through a ketal intermediate: [26] a keto tautomer (initially 7 from
Scheme II) first undergoes the addition of an alcohol across a protonated carbonyl, followed by a deprotonation to give a
hemiacetal; a protonation of the hydroxyl then facilitates its elimination as water, allowing for a renewed C–O π bond, a repeated protonation of
the oxygen, the addition of a second alcohol, and the formation of the ketal through a final deprotonation. However, since a neutral ether can result
via elimination from either an acetal or a hemiacetal, the latter would be more likely owing to the fewer steps involved. Interestingly, the steps of
an acetalization have been shown to occur in a concerted fashion. [27]
[G]: The high consumption of methylating agent is probably due to the presence of the monoether, which is still very
much susceptible to C-alkylation.
[H]: This is a likely origin for the claim, presented by GC_MS on The Hive [cited in the permethylation chapter (III)
of my previous post], where a protective effect against the saponification of dimethyl sulfate by aqueous alkali was ascribed to the presence of 2
mL/g of acetone. Here, however, the authors merely suggest a possibility.
References
[1]: W. Will, K. Abrecht (1884): Ueber einige Pyrogallussäure- und Phloroglucinderivate und die Beziehungen derselben zu
Daphnetin und Aesculetin.
https://doi.org/10.1002/cber.188401702110
[2]: A. Shulgin, A. Shulgin (1991): PiHKAL. (#162 TMA-6)
https://isomerdesign.com/PiHKAL/read.php?id=162
[3]: J. Herzig, F. Wenzel (1906): Studien über Kernalkylierung bei Phenolen.
https://doi.org/10.1007/BF01525129
[4]: A.R. Stein (1964): THE METHYLATION OF THE RESORCINOLATE DIANION.
https://doi.org/10.1139/v65-200
[5]: J. Herzig, B. Erthal (1910): Notiz über die Darstellung des Hexa- und Pentamethylphloroglucins.
https://doi.org/10.1007/BF01530256
[6]: H. Kawamoto, F. Nakatsubo, K. Murakami (1996): O-BENZYLATION OF PHLOROGLUCINOL VIA PHLOROGLUCINOL TRIACETATE.
https://doi.org/10.1080/00397919608003645
[7]: A. Baeyer (1886): Ueber das Trioxim des Phloroglucins.
https://doi.org/10.1002/CBER.18860190145
[8]: J.W. Baker (1934): Tautomerism.
https://doi.org/10.1038/135247a0
[9]: J. Thiele (1899): Zur Kenntniss der ungesättigten Verbindungen. Theorie der ungesättigten und aromatischen
Verbindungen.
https://doi.org/10.1002/jlac.18993060107
[10]: F.E. Ray (1934): Thiele's Theory of Partial Valency in Terms of Electrons.
https://scholarworks.uni.edu/pias/vol41/iss1/43/
[11]: A. Bayer (1885): Ueber die Synthese des Acetessigäthers und des Phloroglucins.
https://doi.org/10.1007/BF01518276
[12]: H. Köhler, G. Scheibe (1956): Übergänge zwischen aromatischen und aliphatischen Formen bei Benzolderivaten.
https://doi.org/10.1002/zaac.19562850315
[13]: A.J. Kresge, G.W. Barry, K.R. Charles, Y. Chiang (1962): The Protonation of Phloroglucinol and its Ethers: An Exception to
the Acidity Function Concept.
https://doi.org/10.1021/ja00881a028
[14]: R.J. Highet, T.J. Batterham (1964): The structure of the phloroglucinol dianion.
https://doi.org/10.1021/jo01025a501
[15]: M. Lohrie, W. Knoche (1992): Dissociation and Keto-Enol Tautomerism of Phloroglucinol and Its Anions in Aqueous
Solution.
https://doi.org/10.1021/ja00056a016
[16]: A. Gissot, A. Wagner, C. Mioskowski (2004): Buffer-induced, selective mono-C-alkylation of phloroglucinol: application to
the synthesis of an advanced intermediate of catechin.
https://doi.org/10.1016/j.tet.2004.06.026
[17]: H. Bredereck, I. Hennig and W. Rau (1953): Alkylierungen mehrwertiger Phenole mit Dialkylsulfat.
https://doi.org/10.1002/cber.19530860909
[18]: M.B. Smith, J. March (2007): March’s Advanced Organic Chemistry.
(6th edition; p. 29, 513–519)
[19]: H.O. House (1972): Modern Synthetic Reactions.
(2nd edition; p. 509–529)
[20]: F. Kaufler (1900): Über den Einfluss der eintretenden Radicale auf die Tautomerie des Phloroglucins.
https://doi.org/10.1007/BF01526082
[21]: E. Deme (1976): Synthesis of Authentic Tri-O-benzylphloroglucinol.
https://doi.org/10.1021/jo00885a031
[22]: K. Freudenberg (1920): Über Phloroglucin Gerbstoffe und Catechine.
https://doi.org/10.1002/cber.19200530818
[23]: J.W. Clark-Lewis (1957): PREPARATION OF PHLOROGLUCINOL TRIMETHYL ETHER.
https://doi.org/10.1071/CH9570505
[24]: W. Will (1888): Ueber einige Reactionen der Trimethyläther der drei Trioxybenzole und über die Constitution.
https://babel.hathitrust.org/cgi/pt?id=hvd.cl1hzt&seq=65...
[25]: J. Pollak (1897): Einiges über die Äther des Phloroglucins und eine Synthese des Hydrocotoins.
https://doi.org/10.1007/BF01518276
[26]: J.C. Touchstone, J. Ashmore, M.N. Huffman (1956): Ethers of Phloroglucinol.
https://doi.org/10.1021/ja01602a049
[27]: E. Grunwald (1985): Reaction mechanism from structure-energy relations. 2. Acid-catalyzed addition of alcohols to
formaldehyde.
https://doi.org/10.1021/ja00302a019
[a4] 3,5-dimethoxyphenol
Also called: Phloroglucinol dimethyl ether
154.16 g/mol
Like so many others, I also opted for the procedure outlined in PiHKAL. [1] At the time of my
experimentation (May, 2022), it never occurred to me to attempt to purify this product, and the fact that it would form crystals became clear only
when composing this post. Removal [or enhanced conversion (via completely trapping/removing all water past the hemiacetal formation, or by repeating
the acetalization procedure)] of the monoether should work to minimize the portion of methylating agent that is wasted on C-alkylation in the next
step; quite possibly improving the yield.
[1]: PiHKAL: #162 TMA-6. https://isomerdesign.com/PiHKAL/read.php?id=162
Experiment 1
Phloroglucinol dihydrate (12.99 g, 80 mmol) was dehydrated to constant weight in a 100°C oven [1] and dissolved in methanol (42 mL) in a 100 mL conical flask. With good magnetic stirring, concentrated
sulfuric acid (7.1 mL, 131 mmol) was added from a dropper, causing the mixture to heat up to 45–50°C and take on a vibrant red coloration. After
first stirring it at room temperature for two hours, the mixture was refluxed for ~8 hours and then allowed to cool.
[1]: Admittedly, this step (on its own) was probably unnecessary; with the excess of alcohol that was used, the reaction doesn't
appear to be that sensitive to moisture.
[Fig. 4] Commercial phloroglucinol dihydrate

[Fig. 5] Reaction mixture following acid addition

Work-up
The cooled reaction mixture was diluted with 65 mL of water. Two extractions were done using 25 mL portions of
dichloromethane. The pleasant-smelling [1] extract was dried over some calcium chloride and
filtered to remove the solids. This was then stored in the dark, at room temperature, for 14 days, after which it was concentrated; first by
distilling it down to half its original volume, and finally by stirring the remainder magnetically on a 45°C hotplate while argon was gently blown
into the flask to carry out the residual solvent. A clear, viscous red oil remained, weighing 7.89 g.
[1]: The odor of the extract was a mixture of floral indole and synthetic wild strawberry essence. There was a definite shared
resemblance to methyl salicylate and (low vapor concentrations of) methyl anthranilate in terms of sweetness and texture.
[b5] 1,3,5-trimethoxybenzene
168.19 g/mol
With the foretold red oil in hand, I thought it the perfect opportunity to revisit the solvent free methylation [1] whose first replication involving the solid methylhydroquinone substrate (a3) was less than representative.
[1]: https://patents.google.com/patent/US4065504A/en
Experiment 1
To the 7.89 grams of red oil from the previous step, there was added potassium carbonate (39.49 g, 286
mmol), [1] followed by dimethyl sulfate (30.1 mL, 318 mmol), and the vessel was equipped with a
water-cooled 200 mm Liebig condenser, which was topped with a bulbous anti-splash adapter. The mixture was stirred magnetically, its temperature
remaining at ~26°C with no external heat being applied; its mobility improved over ~20 minutes as the beads of base grew less clumped together.
Heating was commenced after the first hour to counteract a thickening consistency, bringing the mixture to ~35°C on a 60°C hotplate. The gradual
addition of water was also deemed appropriate at this time, and was begun, five drops at a time. [2] 20 minutes later (at the 90-minute mark), 1.4 g of water had been added. The reaction temperature had reached 41°C, and
the hotplate setting was raised to 80°C. Very suddenly, the rate of the reaction picked up dramatically, and the reaction temperature shot up to
75°C, by which point the heating was discontinued; dense fumes were observed exiting the apparatus, as the peachy gruel in the flask below curdled
into a white, clumpy porridge from which a clear, red liquid phase separated. [3] Larger,
approximately half-gram portions of water were added to just barely keep the stir bar functional. [4] Heating was resumed once the mixture had cooled to 66°C. Two hours into the reaction, a thinner, more homogenous
consistency had been regained; 7 g of water had been added, and the internal temperature was a stable ~60°C on the 100°C setting — and yet, a slow
thickening persisted, which prompted the addition of three more grams of water. Finally, the mixture was kept agitated for a little over 15 more
hours, with the hotplate set to 125°C.
[0]: The temperatures were measured from the exterior surface of the flask using IR.
[1]: This time, the carbonate had been briefly ground down to a slightly finer consistency.
[2]: 10 g of water had been pre-measured for use as a lubricant in near-accordance with the patent that was followed; the
instructions are to use an amount that is "at most equal to, but usually considerably less than, the initial weight of the hydroxybenzene compound".
In hindsight, I do not believe that 7.89 grams would have been sufficient here — but then, I suspect that the embodying reactions of the patent were
stirred mechanically.
[3]: I suspect that this sudden exothermic eruption was mostly due to C-alkylation of the monoether, to which the added water would
seem likely to have contributed. Moreover, the observed generation of chromophores and the persisting reddish oil (during workup) would match
literature descriptions of the C-alkylated pseudoethers.
[4]: Although the stir bar spun very fast and never decoupled, the parts of the mixture that had risen up against the sides of the
flask stagnated momentarily as the reaction hit the peak of its vigor. Barring mechanical stirring, a larger (high-torque) stir bar could avoid this
problem, particularly if powdered K2CO3 was added to a stirred mixture of the liquid components in the beginning (however, the
presence of the monoether bears consideration). One might also be more liberal in their use of the allocated water early on, and consider using a
water bath that can act as a heat sink such that there is more time to react, should the reaction suddenly sustain itself and then some. Having said
all that, a second attempt by me would avoid the hassle and just use acetone as solvent.
[Fig. 6] Addition of potassium carbonate to crude diether

[Fig. 7] 30 minutes after DMS addition

[Fig. 8] 1:15 into the reaction

[Fig. 9] (1:38) Sudden increase in reaction vigor

[Fig. 10] (1:52) Immediate aftermath
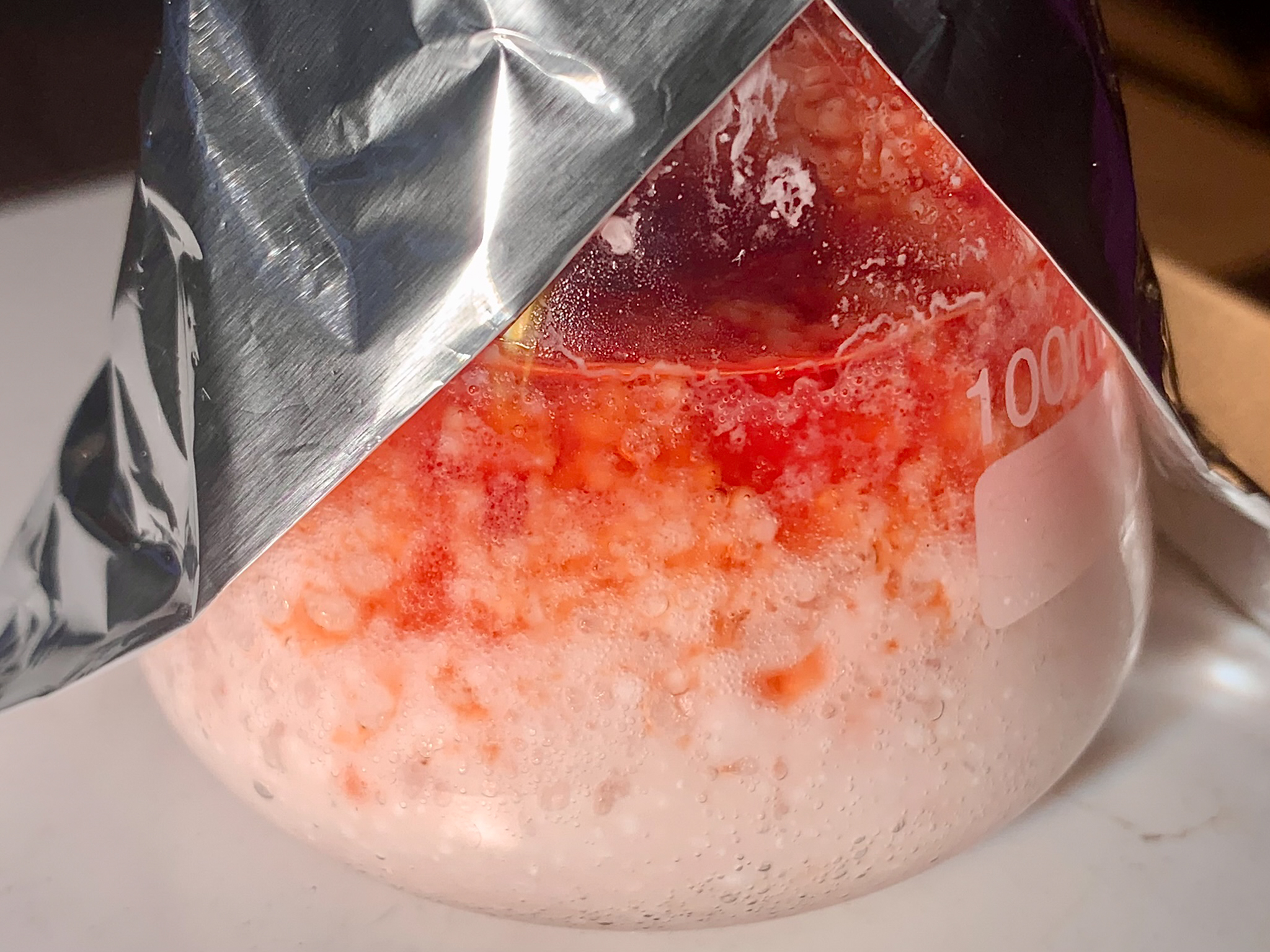
[Fig. 11] Reaction mixture following complete water addition

Work-up
On the following day, 50 mL of water was used to dilute the mixture, which resulted in the separation of three
distinct phases; a layer of mostly solid sulfate accumulated at the bottom of the flask, whereas the buff, water-insoluble crude product floated to
the top, leaving a pale salmon-colored suspension between the two. The mixture was vacuum filtered, and most of the salmon-buff top layer of the cake
was scraped into a beaker where it was dissolved in 10 mL of DCM. The remaining solids were transferred to a second beaker and stirred with three
consecutive 10 mL portions of DCM; the last of these was used to rinse the Büchner funnel and to extract the aqueous filtrate, which was then
extracted with one or two more such portions. The organic extracts were pooled and washed with 10 mL of 10% aqueous NaOH, which gave rise to an
emulsion that was resolved by a few additional grams of water.
The solvent was removed by first distilling the mixture under atmospheric pressure, followed by some moments under vacuum, and then by magnetically
stirring the residue under a steady discharge of argon. The oily, amber residue was cooled down close to 0°C in the hopes of observing
solidification, but there was none. 10 mL of heptane was added to give a solution with a light dusting of insolubles in it. The solid impurity was
removed via filtration through a dense ball of cotton, which was then rinsed using a dash of fresh heptane. The solution was evaporated to dryness at
room temperature, resulting in 6.67 g of a surprisingly hard, amber, crystalline solid with a phenolic, slightly woody, earthy (not moldy), medicinal,
artificial strawberry odor subtly hinting at vanilla. After melting the solid in a 50°C oven, 6.62 g remained, and had a melting point of
42.3–48.5°C
The 6.62 g of crude product was recrystallized from 19.1 g of 78.5% MeOH to obtain 5.7 g of crystals with the appearance of Himalayan rock salt and a
melting point of 51.1–52.3°C. A second recrystallization from 15.9 g of the solvent gave 5.26 g of material with less discoloration, no odor, and a
slightly higher melting point of 51.7–53.0°C; this is what was used in the subsequent formylation.
An impure residue from evaporating the mother liquors was rinsed with 3 mL of ice-cold isopropanol to afford 0.98 g of a reddish material which was
recrystallized from 2.74 g of 81% MeOH but neglected such that it evaporated nearly to dryness. Most of the impure liquor was removed by pressing the
material between folded paper towels, yielding 0.86 g of dull-brown crystals — for an unexciting total yield of no more than 45.5%, based on
phloroglucinol.
[0]: According to literature, the 1,3,5-trimethoxybenzene can be purified through steam distillation; despite reports of the neutral
C-alkylated side products also coming over with steam, it would be interesting to see the results from attempting its isolation directly from the
reaction mixture in this way.
[Fig. 12] Reaction mixture following overnight stirring

[Fig. 13] Dilution with water

[Fig. 14] Vacuum filtration

[Fig. 15] Evaporating solution of crude product in heptanes

[Fig. 16] Crystalline evaporation residue

[Fig. 17] First recrystallization

[Fig. 18] Recrystallized product

[Fig. 19] Second recrystallization
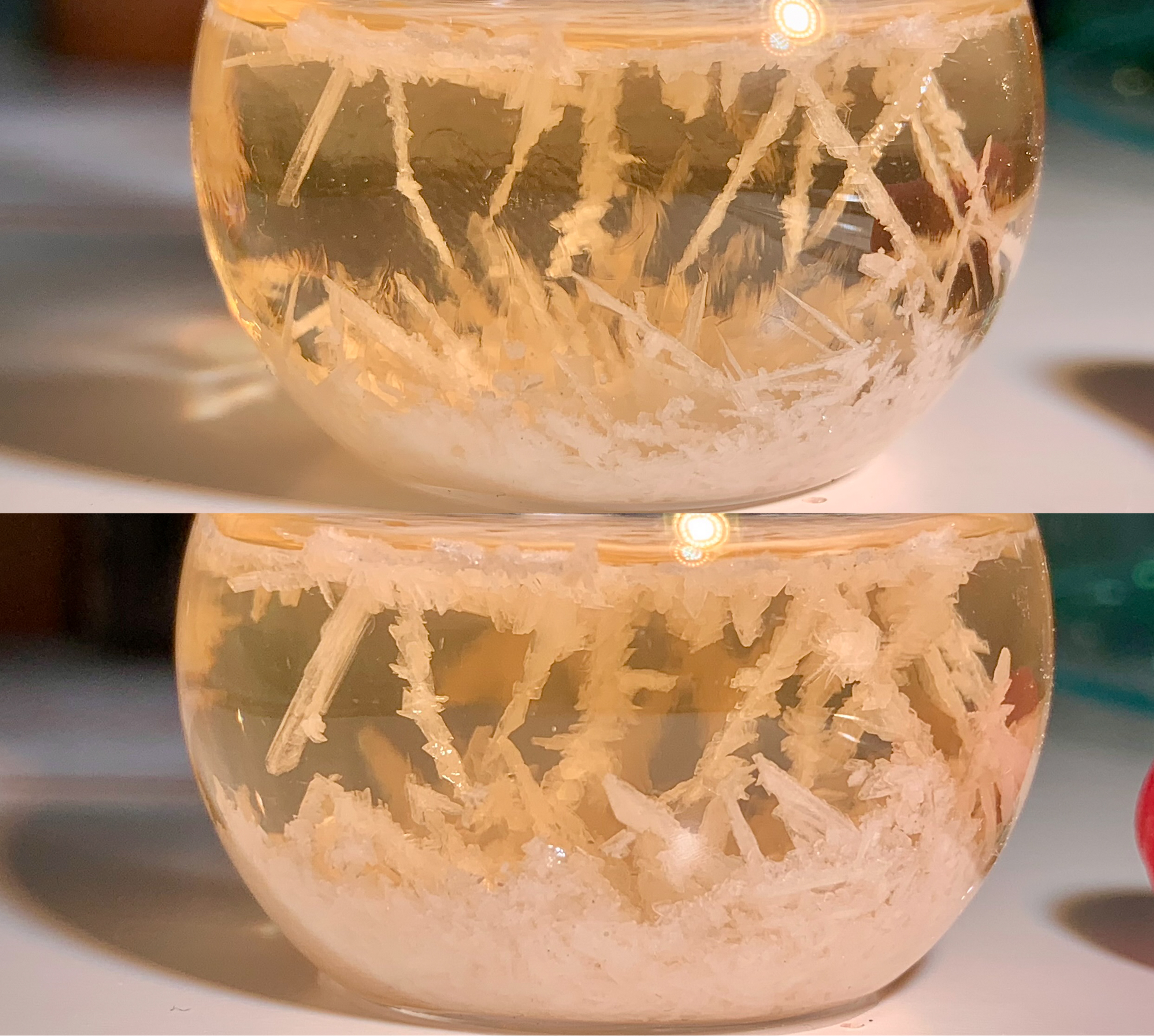
[c4] 2,4,6-trimethoxybenzaldehyde
196.20 g/mol
316.37 g/mol (bisulfite adduct; potassium salt)
A deviation from the PiHKAL procedure, this experiment substitutes N,N-dimethylformamide for N-methylformamide, because the latter is reported to
produce dramatically inferior yields of an impure product. [1] [2] Indeed, just as originally
reported by the Hyperlab member _hest, [3] this modification gave excellent results.
[1]: PiHKAL: #162 TMA-6. https://isomerdesign.com/PiHKAL/read.php?id=162
[2]: (Hyperlab) amethyst: TMA-6. https://hyperlab.info/inv/index.php?s=&act=ST&f=17&a...
[3]: (Hyperlab) _hest: Synt of 2,4,6-trimethoxybenzaldehyde. https://hyperlab.info/inv/index.php?s=1f1c32485b7603b280beee...
Experiment 1
In a round 50 mL reaction flask, 1,3,5-trimethoxybenzene (5.00 g, 29.7 mmol) was dissolved in 20 mL of DMF. A stir
bar was added, and the mixture was set up in a stationary ice bath over a stirrer. A pasteur pipette was driven through a thermometer adapter to
suspended it above the magnetically agitated mixture; through this, there was gradually injected phosphoryl trichloride (4.87g, 31.8 mmol) at a
fluctuating dropwise pace over 40 minutes. [1] Two hours after completing the addition, the
now-orange mixture was removed from the bath, whose temperature of 9°C it shared. Stirring was resumed for 2.5 hours at 24°C.
[1]: This arrangement served as an improvised way of limiting air exchange inside the flask (to protect the POCl3) in lieu
of an appropriately sized addition funnel, and worked passably: the opening was otherwise airtight, which necessitated the application of some
pressure from the dropper in hand; hence, a thin bypass through the O-ring would have been shrewd. On the other hand, the consequences of simply
alternating a stopper instead might have been negligible.
[Fig. 20] Discolored 1,3,5-TMB dissolving in DMF

[Fig. 21] Reaction apparatus

[Fig. 22] Claret hue evolving from initial additions

[Fig. 23] Reaction mixture following removal from bath

Work-up
Approximately 150 mL of cool, clean, well-stirred water from the tap was prepared in a 250 mL beaker; the contents
of the reaction flask were then added to this in <10 minutes using a dropper, causing the temperature of the mixture to rise to ~30°C before
gradually cooling down again. A sudden onset of precipitation was observed after 20 minutes. The mixture was allowed another 8 hours of stirring (in
the dark, although this is probably not important) prior to a vacuum filtration to separate the solids, which were rinsed with six 10 mL portions of
water and air-dried to obtain 5.26 grams (90.2%) of a delightfully pink, [1] crystalline solid
melting at 119.5–120.5°C.
[1]: The pink hue seems to have almost completely faded away over time, doing so much faster, initially.
[Fig. 24] Post-reaction mixture being added to water

[Fig. 25] Completed quenching mixture after some minutes of stirring

[Fig. 26] Quenching mixture after 35 minutes of stirring

[Fig. 27] Mixture prior to vacuum filtration

[Fig. 28] 1,3,5-trimethoxybenzaldehyde

This at last concludes the benzaldehyde portion of my quest for the corresponding phenethylamines!
As for what's next, I'm pleased to announce that I have made some progress on the remainder as well: Having decided (in light of some initial failures
which had fascinating implications) that it would be apt to learn more about the condensation and subsequent reduction in order to minimize the
further waste of precious starting materials, I was able to prepare all of the intended nitrostyrenes, which are currently pending reduction as I'm
figuring out the copper-catalyzed process that I quite prefer over the alternatives at my disposal. I feel like I'm finally getting close to where I
want to be, although a good deal of work still remains to be done; but I do believe that it'll all be worth it.
Whoever you are, whenever you are: thank you for making it here !




















 you would be better
off eating saccharin than you would succinimide.
you would be better
off eating saccharin than you would succinimide.






















































































































































 - but you wouldn't happen to have noted the yield you got from the NBS bromination, have you ?
- but you wouldn't happen to have noted the yield you got from the NBS bromination, have you ?
































































































































































































































 )
)



































































































































































