palico
Hazard to Self
 
Posts: 59
Registered: 1-10-2013
Member Is Offline
Mood: No Mood
|
|
Synthesis of benzene from PET water bottles
Hello people,
today I present a very useful preparation, the synthesis of benzene from PET water bottles. The reaction employed is the pyrolysis of PET pieces with calcium hydroxide. The raw product is then purified by
distillation. According to the reference [1], it proceeds through the initial PET hydrolysis to calcium terephthalate which then decarboxylate to benzene. Toluene, ethylbenzene, xylene
and others are found as side-products.
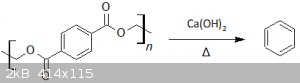
Procedure
The synthesis is realized in a 11.4 l steel alcohol distiller re-adapted as reactor, purchased from eBay. PET bottles are cleaned and eventually
dried, removed of label, cap, seal, also top and bottom part because too much hard, and cutted in pieces. Then 0.5 kg of PET pieces is mixed with 1.5
kg of calcium hydroxide ( PET:Ca(OH)2 1:3 ), introduced into reactor, pyrolysis started on a gas burner flame. The setup is in Figure.
 
The flame is open to maximum, the reactor bottom is red hot. Temperature of pyrolisate overcome 200 Celsius. Some fumes are collected initially, but
then a yellow-green liquid pass through condenser along with water and wax. After a total of 2 hours, heating is interrupted, and everything let cool
down to room temperature. Pyrolisate is separated from aqueous layer, extracted with 3x50 ml of water. Organic phase filtered through cotton plug to
remove as much wax as possible. A yellow-green clear liquid is obtained which quickly turn brown; crude pyrolisate is 45 g ( 47 ml ), corresponding to
9% of the initial PET mass.
After collecting five batches, 250 ml of that is distilled simply in oil bath at 100 Celsius, discarding the fraction passing below 75 Celsius and
collecting the one between 78-80 Celsius; 95 ml of very clear limpid benzene is obtained, d20 = 0.870 g/ml.

Discussion
Almost 100 ml of very pure benzene are collected from 2.5 kg of PET bottles. Reference report that from 1:5 PET:Ca(OH)2 ratio on, almost pure benzene
is obtained, so theoretically the yield could increase much more.
Anyway, this experiment demonstrate that very valuable chemicals can be got from a trash material as water bottles. The reaction should not be seen
only as a benzene synthesis but also the chemical recycling of PET bottles, which otherwise have to be done mechanically. The higher fractions can be
recoverd as well, adding value to the experiments.
I think this reaction can be scaled up to become economically sustainable. Gas can be recovered and used to fuel the reaction itself, which furtherly
decrease cost.
If biomethane is used as fuel, that add renewability to the process.
Reference
1. Chem. Lett. vol. 33, pp. 282-283, 2004
As usual, I thank you for the attention and remind you to watch the detailed procedure on my YT channel. Here you can find synthesis, purification, flammability test.
palico
|
|
|
dettoo456
Hazard to Others
  
Posts: 178
Registered: 12-9-2021
Member Is Offline
|
|
Nice job. The yield is kind of shit considering the weight of PET required and natural gas but it’s definitely worth it if benzene is harder to get
near you. You can also pyrolize HDPE & LDPE w/ alumina to yield long chain alkanes with potential use in gas engines.
A smaller reaction vessel made out of steel and shoved into a kiln (or just a better insulated heat source) might be more economical and achieve
higher yields as compared to the stove.
|
|
|
Fery
National Hazard
   
Posts: 990
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Well done and quite big scale experiment!
Ca salts of organic acids are used for producing ketones. I wonder whether using Na terephthalate couldn't yield more benzene or whether you also did
not obtain substantial amounts of benzophenone.
|
|
|
palico
Hazard to Self
 
Posts: 59
Registered: 1-10-2013
Member Is Offline
Mood: No Mood
|
|
@dettoo456: the yield can be increased with higher PET:Ca(OH)2 ratio according to reference. I could not use 1:10 because the volume of reactor but
1:5 already gave much pure pyrolisate, almost wax free. Is wax which lower the yield. Filtration takes long time, during the which pyrolisate
evaporate quite a lot.
@Fery: other compounds are in distillation residure. But I did not separated.
|
|
|
Hexabromobenzene
Hazard to Self
 
Posts: 99
Registered: 27-4-2021
Member Is Offline
|
|
Have you had problems with clogged pipes? When pyrolyzing PET bottles WITHOUT calcium oxide, a large amount of sublimate is formed from benzoic and
terephthalic acid (mainly terephthalic). This leads to clogging of pipes and explosion of the pyrolysis apparatus in extreme cases
But if you assemble a pyrolysis apparatus that will not clog, then you can make large quantities of benzoic and terephthalic acid for free. Benzoic
acid is separated from terephthalic acid by hot water extraction
A lot of acetaldehyde is formed from by-products. Terephthalic acid can also be contaminated with biphenylcarboxylic acid and alkylbenzoic acids
I feel very sorry for this distillation apparatus. Once the plastic is pyrolyzed, it will become contaminated. Better to use a paint can
[Edited on 26-1-2024 by Hexabromobenzene]
|
|
|
Hexabromobenzene
Hazard to Self
 
Posts: 99
Registered: 27-4-2021
Member Is Offline
|
|
Quote: Originally posted by dettoo456  | Nice job. The yield is kind of shit considering the weight of PET required and natural gas but it’s definitely worth it if benzene is harder to get
near you. You can also pyrolize HDPE & LDPE w/ alumina to yield long chain alkanes with potential use in gas engines.
A smaller reaction vessel made out of steel and shoved into a kiln (or just a better insulated heat source) might be more economical and achieve
higher yields as compared to the stove. |
You can use firewood and wood wastes
|
|
|
palico
Hazard to Self
 
Posts: 59
Registered: 1-10-2013
Member Is Offline
Mood: No Mood
|
|
@hexabromobenzene: yes, acetaldehyde odor is detected at the beginning of pyrolysis. No, I have never pyrolyzed PET without catalyst, and I will not.
This apparatus is dedicated to PET pyrolysis only, do not feel sorry.
|
|
|
ItalianChemist
Hazard to Others
  
Posts: 172
Registered: 26-1-2011
Location: Italy
Member Is Offline
Mood: No Mood
|
|
Very nice setup! Well done!
The final product looks very pure!
|
|
|
dettoo456
Hazard to Others
  
Posts: 178
Registered: 12-9-2021
Member Is Offline
|
|
Moved from other thread:
This paper (https://doi.org/10.1016/j.polymdegradstab.2022.109900) which describes PET pyrolysis to yield Paraldehyde, EG, and Benzoic Acid under fairly
‘weak’ conditions. Those conditions being atmospheric pressure, no catalyst or pH modifiers, and fairly fast (although they say slow) heating to
only 400C. The whole process would only take 13 minutes apparently; 40C min−1 to 200C followed by 25C min−1 to 400C. They don’t state a dwell
time at the 400C as far as I could tell, but in any case it wouldn’t be too difficult to replicate in theory.
A yield of around 24g Paraldehyde, 10.5g Ethylene Glycol, & 4.6g Benzoic Acid are recovered as “oil” from 100g of PET.
I’m looking to maybe try this procedure out in an Al paint pressure canister modified as a retort. Does anyone have any experience with a prep
similar to this (that being PET pyrolysis at lower temps without any catalyst)?
|
|
|
palico
Hazard to Self
 
Posts: 59
Registered: 1-10-2013
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by dettoo456  | Moved from other thread:
This paper (https://doi.org/10.1016/j.polymdegradstab.2022.109900) which describes PET pyrolysis to yield Paraldehyde, EG, and Benzoic Acid under fairly
‘weak’ conditions. Those conditions being atmospheric pressure, no catalyst or pH modifiers, and fairly fast (although they say slow) heating to
only 400C. The whole process would only take 13 minutes apparently; 40C min−1 to 200C followed by 25C min−1 to 400C. They don’t state a dwell
time at the 400C as far as I could tell, but in any case it wouldn’t be too difficult to replicate in theory.
A yield of around 24g Paraldehyde, 10.5g Ethylene Glycol, & 4.6g Benzoic Acid are recovered as “oil” from 100g of PET.
I’m looking to maybe try this procedure out in an Al paint pressure canister modified as a retort. Does anyone have any experience with a prep
similar to this (that being PET pyrolysis at lower temps without any catalyst)? |
That article show another way to get resources from PET. Do you see ? Waste does not exist, but are just resources ?
|
|
|