chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Reaction conditions for amine to R-methyl sulfonyloxy couplings
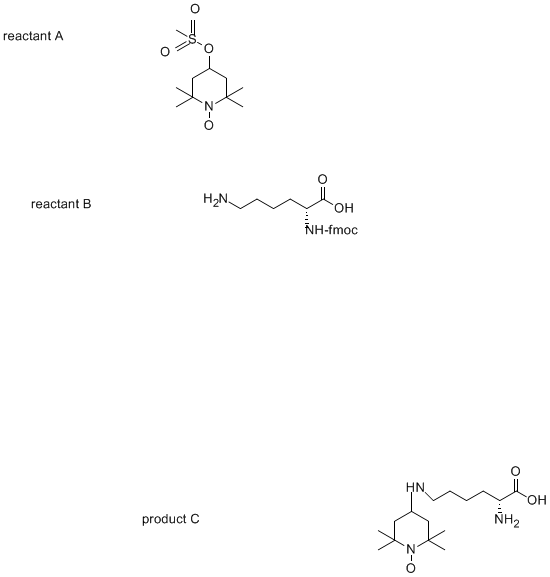
A friend of mine wants to perform the reaction (see below), the sulfonyloxy compound contains an N-oxide which is paramagnetic (interesting for NMR).
Anyway this is meant to be a known coupling reaction, but no information could be found about the reaction conditions.
I suggested reflux in DCM or DMF for a few hours... perhaps in the presence of an unreactive base (triethylamine?) to keep the reaction basic so that
the amine remains -NH2 and is not turned into -NH3+...
Any thoughts?
The first compound is the tetramethyl (methylsulfonyloxy)-1-piperdinooxy radical (googling doesn't help though), and the second is Fmoc-Lysine - with
a free side chain. The Fmoc must remain on the lysine (as product 3 is used for peptide synthesis), so a strong base such as piperidine cannot be used
as the Fmoc would be cleaved off otherwise).
[Edited on 27-4-2008 by chemoleo]
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
This is not going to be easy at all. I'm afraid your colleague might have chosen a not so ideal synthetic approach. The reasons are in that cyclic
alkyl mesylates (or psudohalides in general) are not particularly reactive for SN2 substitutions, they are even less reactive than sec-alkyl mesylates
(or psudohalides in general). Not just the slow reaction, but also the competition with elimination is one of the resulting problems. I also have no
idea how the free radical influences the conditions needed. The Fmoc protection might not survive the basic condtions at the temperature required and
the carboxylic group should be protected since it will get deprotonated by the Hunig's base needed as MeSO3H scavenger and thus behave as a competing
nucleophile. Are you sure that this was the synthetic approach someone used before? If yes, then writing the authors of the paper where the product is
used would be the first choice, before even starting to loose time and material with finding the conditions required. Otherwise, I think NMP could be
a good solvent for this alkylation, or maybe sulfolane. I think heating to >50°C would be necessary, probably 80-120°C (only following with TLC
can tell you what the minimum T for the reaction is).
If I would be given the job to make that end compound, I would rather try reductively aminating the 4-oxo-TEMPO with Fmoc-lysine using sodium
triacetoxyborohydride (STAB; there is a recent thread with a literature review). This way you skip any problems with possible lack of reactivity of
the 4-mesyloxy-TEMPO, potential need for COOH protection and Fmoc protection instability toward bases/heat. The only major drawback I can think of at
the moment is in that I'm unsure if the nitroxyl radical survives the reduction with STAB. It might easily get reduced to the hydroxylamine
counterpart (thus adding an oxidation as the additional step in the synthesis). But at least such experiment would use only commercial starting
materials and wouldn't take much effort.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Reductively aminating 4-oxo-TEMPO is a pretty good idea. There are several articles on this in the literature, with varying substrates. Most of the
time good to very good yields are obtained IIRC.
Depending on what reagents he can obtain (is the mesyl-TEMPO a commercial product?), it could be easier to directly start from distilled
4-oxo-TEMPO, than to reduce it and and form the sulfonate.
What kind of uses will be end product be applied to? Just curious.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Indeed, apparently it can be done…
Reductive amination of 4-oxo-TEMPO with a primary alkyl amine and STAB:
Bioorganic & Medicinal Chemistry Letters, 17 (2007) 1451-1454. DOI: 10.1016/j.bmcl.2006.11.040
Using NaBH3CN:
European Journal of Organic Chemistry (2002), 1912-1918.
Tetrahedron, 41 (1985) 1165-72;
Synthesis (1984), 125-126.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Thank you very much for the input.
The presence of the unpaired electron makes the TEMPO probe paramagnetic, which has applications in NMR where it serves as a probe once again. This is
then used for structural elucidation of the peptide binding to a target protein.
The idea is that the modified TEMPO lysine can be incorporated into a peptide sequence during peptide synthesis, using standard Fmoc chemistry, where
TEMPO-lysine is treated like any other amino acid.
Alternatively it could be added to a minimal medium for E.coli growth which contains an expression plasmid for the desired protein sequence, in the
hope that the TEMPO-lysine is incorporated. I don't think that approach will work however, as numerous reactive/reductive processes occur within
living cells - i.e. the radical wouldn't survive.
Anyway, another idea I had was to use carboxy-TEMPO,
http://www.sigmaaldrich.com/catalog/search/ProductDetail/ALD...

which could be coupled straight to the side chain amino group of the lysine (whilst Fmoc remains on) using the standard peptide synthesis coupling
reagents, i.e. PyBOP, HOBt and DIPEA....
What do you think of this?
4 oxo-TEMPO seems like a good choice too, good idea, even found it at Sigma... but why do you talk of 'reductive' amination?
I could simple form the imine/Schiff's base, I don't think this would be an issue other than the steric hindrance one...
Using these precursors, what do you think the best reaction conditions are? I'd think pretty much as above, i.e in DMF or NMP, at RT for a couple of
hours? Not sure about the suitability of MeOH due to solubility problems with the Fmoc lysine.
The lysine side chain (NH2-(CH2)4) is an aryl so it should be stable with the imine group at its end, right?
I think I'd have to dig out that Kaiser test to make sure no free amine remains.... trouble is, this assay is almost too sensitive, so it is hard to
tell whether the reaction is 50% complete or 99% complete.
Or perhaps look for the disappearance of the carbonyl...wasn't dinitrophenylhydrazine a choice reagent for this? No more precipitate, no more carbonyl
remains...I've never done that test though.
[Edited on 30-4-2008 by chemoleo]
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
sparkgap
International Hazard
    
Posts: 1234
Registered: 16-1-2005
Location: not where you think
Member Is Offline
Mood: chaotropic
|
|
| Quote: |
Alternatively it could be added to a minimal medium for E.coli growth which contains an expression plasmid for the desired protein sequence, in the
hope that the TEMPO-lysine is incorporated. I don't think that approach will work however, as numerous reactive/reductive processes occur within
living cells - i.e. the radical wouldn't survive.
|
Neither do I. It looks too different from good ol' lysine (largish pendant group and all) that I don't think E. coli will be tricked into using it as
a replacement for lysine.
As to coupling the carboxy-TEMPO, I can't say with certainty that the radical function will withstand the conditions; maybe it should be tried out in
small-scale? I've seen nothing from a cursory search of the usual refs.
| Quote: | | perhaps look for the disappearance of the carbonyl...wasn't dinitrophenylhydrazine a choice reagent for this? No more precipitate, no more carbonyl
remains...I've never done that test though. |
That might be OK. 2,4-dinitrophenylhydrazine is quite soluble in DMF (maybe in NMP, too?)
sparky (~_~)
"What's UTFSE? I keep hearing about it, but I can't be arsed to search for the answer..."
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
I'm not sure using DNPH for monitering imine formation would be possible, the formation of the hydrazone would surely be prefered over the imine,
displacing the equilibrium.
On the other hand, during the reduction of the imine, it could be used, no more imine, no more carbonyl in equilibrium, no more hydrazone.
It's a pity i don't know much in biochemistry, it seems like a very complexe and interesting science.
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
sparkgap
International Hazard
    
Posts: 1234
Registered: 16-1-2005
Location: not where you think
Member Is Offline
Mood: chaotropic
|
|
On my part, I was counting on the coupled product to be insoluble in the reaction solvent to be easily separated, and then one does the testing on the
solution with 2,4-DNPH to check for unused carbonyl. Of course, my assumption could be invalid... in which case Klute's point is valid; you don't want
competition on this one.
sparky (~_~)
"What's UTFSE? I keep hearing about it, but I can't be arsed to search for the answer..."
|
|
|