Jor
National Hazard
   
Posts: 950
Registered: 21-11-2007
Member Is Offline
Mood: No Mood
|
|
Dissolving gold in KCN-solution
30 minutes ago, in the fume hood with extreme caution, I took a spatula full of potassium cyanide, and added this to a test-tube. To this, 2mL of
water was added, followed by 2 drops of 30% KOH. Next, a small pinch of gold powder (I refined this from dental gold, should be at least 99%) was
added, and I vigorously shaked the liquid, to allow acces for oxygen. After 2 minutes there was still no yellow color, wich is very obvious, even at
low concentrations of gold in solution. The Au(CN)2- ion is yellow, just like the AuCl4- ion, right?. I thought the gold should be very readily
oxidised by oxygen, wich should dissolve fairly well in water at these temperatures (about 5-10C).
Can I just add H2O2, to speed things up? I'm not sure if H2O2 will oxidise CN- to OCN- ... I think it will, but maybe the H2O2 reacts with any present
gold first?
I want to experiment with the stability of the cyano-complex. It is said to be VERY stable, so I wanna see if it can withstand sulfuric acid
(non-coordinating acid), even with heating, and how it responds to strong oxidisers , like bleach (although this contains chloride-ions, wich complex
the gold as well, I rather have a non-coordinating oxidiser. Maybe persulphate). Also will gold drop out of solution when I add silver powder, that's
something I want to test, as the silver complex is also very stable.
EDIT: 3 hours later the solution is still colorless. I checked google. dicyanoaurate(I) is supposed to be colorless.
However, I can't see how much gold has dissolved, but it seems it is not much.
[Edited on 15-12-2008 by Jor]
[Edited on 15-12-2008 by Jor]
|
|
|
woelen
Super Administrator
        
Posts: 8149
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Try adding a few drops of the (supposed) gold(I) solution to a dilute solution of a reductor. Even small amounts of gold will produce a nice colloidal
gold solution, with a blue/purple color.
Gold does not simply dissolve in a cyanide solution. You really need to have access to oxygen as well. You could add a single drop of H2O2.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | | The cyanide solution strength is also important in leaching gold, with the typical range of solution being in the 0.02% -0.05% NaCN. The gold particle
size has a tremendous effect on the time required for dissolution in a cyanide solution. Generally, the finer the gold, the quicker it will dissolve.
A 45 micron particle of gold would dissolve in 10-13 hours, while a 150 micron particle might take from 20 to 44
hours to dissolve in the same solution. |
http://www.mine-engineer.com/mining/minproc/cyanide_leach.ht...
Pure gold dissolves more slowly than gold alloyed with small amounts of less noble metals.
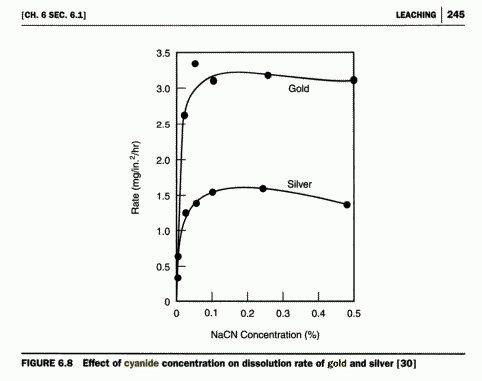
|
|
|
jhullaert
Harmless

Posts: 13
Registered: 11-12-2008
Location: Ghent Belgium
Member Is Offline
Mood: No Mood
|
|
Why is there a peak at the gold rate at 0,05 in the picture?
Is there any reason?
(Woelen and jor: I'm chemaniac  . Don't be afraid I won't spam. . Don't be afraid I won't spam. ) )
|
|
|
Jor
National Hazard
   
Posts: 950
Registered: 21-11-2007
Member Is Offline
Mood: No Mood
|
|
Welcome here chemaniac 
I wonder why dissolution is best at such a low cyanide concentration. Probably to use an as high volume of water as possible, to dissolve as much
oxygen as possible, while having enough cyanide, so the complex is stable.
I am now using a 5-10% solution of KCN! 
I am going to dilute it at least 10 times I think.
PS: KCN appearance is just like that of sugar  . .
|
|
|
woelen
Super Administrator
        
Posts: 8149
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Chemaniac, welcome and good to see you here!! I hope (and expect!) you will enjoy sciencemadness.
I also am surprised to read about the low concentration of cyanide for dissolving gold. I expected it to be at least 10 times as high.
|
|
|
Fleaker
International Hazard
    
Posts: 1254
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
It is used at such concentration to ensure selectivity; note the source of his information, a site dealing with extraction from ores. At low
concentrations, one can selectively strip the gold off of say, a copper substrate (in fact, such a product is sold for such a purpose, technistrip
AU). This low free CN- is really only used for leaching. I assume that you Jor, are not leaching ore, but are trying to dissolve something like bulk
gold into cyanide? If that is the case, then your best bet is to use a concentrated cyanide solution (FYI, sodium cyanide will solvate the gold faster
and the corresponding sodium gold cyanide salt has higher solubility, however, both are great for plating baths) and make sure it is buffered at pH
11. You can use H2O2 as the oxygen source, but you would be surprised at how convenient a fish tank bubbler is! One can easily make a sparging tube
from borosilicate and some glasswool that is loosely packed in the end of the tube.
Let me know how it goes!
Neither flask nor beaker.
"Kid, you don't even know just what you don't know. "
--The Dark Lord Sauron
|
|
|
Jor
National Hazard
   
Posts: 950
Registered: 21-11-2007
Member Is Offline
Mood: No Mood
|
|
Your too late Fleaker 
I diluted 10 times. My guess is a concentration of about 0,5%. How should I buffer it?
I have added 2 drops of 30% KOH at start. But when the gold disoolves, hydroxide is formed. What acid/base mixture should I add? Acetic acid/acetate
buffer? Phosphate/phopshoric acid?
|
|
|
Fleaker
International Hazard
    
Posts: 1254
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
Those have too low of a pH and you may have some hydrolysis to HCN, which is smelly and dangerous. Typically sodium carbonate is used.
You don't need to use KOH, it's a bit strong. 10-11 pH and you're in the clear, so carbonate should be fine.
Neither flask nor beaker.
"Kid, you don't even know just what you don't know. "
--The Dark Lord Sauron
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Actually that chart, from The Chemistry of Gold Extraction John Marsden, was done on sheets of the pure metals IIRK. It was a research
thing, a lot easier to give surface area in square units with flat pieces of metal rather than bits of metal dispersed in crushed ore. So those
numbers do apply to Jor's case, although estimating surface area may difficult.
Many years ago I watched someone clean up silver (fine - 99.9%) medallions using NaCN solution and H2O2. The silver was left in the mix for several
minutes, at which point you could begin to see crystal structure patterns on the surface. The dealer said that so little silver was dissolved that
they'd stopped recovering it as it was uneconomic.
|
|
|