carbon chloride
Harmless

Posts: 12
Registered: 18-8-2009
Member Is Offline
Mood: No Mood
|
|
1,2-diiodo-ethanol?
Does anyone know how one could synthesize this compound? Out of things like iodine, iodomethane, methanediol, ethylene glycol, stuff like that?
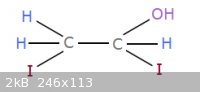
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
In theory, it would be obtainable by equimolar addition of I2 across the double bond of vinyl alcohol, CH2=CHOH. However, vinyl alcohol is unstable,
and spontaneously rearranges to acetaldehyde, CH3-CH=O; with the result that its polymerization product polyvinyl alcohol (used as an adhesive) can
only be obtained indirectly by hydrolysis of its ester polyvinyl acetate. This rearrangement also rules out direct iodination of vinyl alcohol by I2.
Another possibility may be addition of HI to CHI=CHOH, if that precursor could be obtained; but, because both carbons have one H atom each,
application of the Markownikoff rule would result in an isomeric mixture.
|
|
|
kclo4
National Hazard
   
Posts: 916
Registered: 11-12-2004
Location:
Member Is Offline
Mood: No Mood
|
|
Just out of curiosity, what is it you'd want this for?   
Often times in organic chemistry there are multiple ways to a single substance, and one might just be more OTC. You maybe able to buy it.. however.
It seems pretty difficult to make IMO, though, maybe something would be possible though, but just acquiring the chemicals to make that look pretty
obnoxious.
|
|
|
carbon chloride
Harmless

Posts: 12
Registered: 18-8-2009
Member Is Offline
Mood: No Mood
|
|
Interesting John, thanks. Perhaps then maybe add I2 to vinyl acetate and then like decarboxylate it...?
Just a stupid idea I've had kclo4, you might have seen it on the vespiary (although I kind of ashamed myself over there don't know if I should go
back)...
Idea is for every mole of this, add two moles of benzene which I would imagine should each replace one of the iodines... then convert the -OH to -Cl
or -I (don't know if it's actually necessary) and add dimethylamine to get lefetamine (a drug). 
|
|
|
kclo4
National Hazard
   
Posts: 916
Registered: 11-12-2004
Location:
Member Is Offline
Mood: No Mood
|
|
I thought that might be you, with such a similar molecule and drawing, etc
You probably just want to make sure you use references or something, show that you've made an attempt to see if the reactions are even possible, or
ask what sort of reaction would be needed to get from say..a benzene and an alkyl iodide to an alkylbenzene, etc.
How is it you propose the benzene will react with this compound? The HI that would theoretically be released could react with the secondary alcohol
formed - which, if it is in some situation where iodides react with benzene, you might get some nasty unwanted products.
|
|
|
carbon chloride
Harmless

Posts: 12
Registered: 18-8-2009
Member Is Offline
Mood: No Mood
|
|
Who is this, Vesp? lol i don't know
That picture was wrong by the way it would never work, but for some reason I'm sure that this would... I would think that each R-I should react with a
benzene to make R-C6H5 + HI, you know?
The only byproduct I can really see is if that hydroxy group does something, which I don't think it will without adequate heat or pressure or
catalyst... I guess it could react with HI but I think that it would need a catalyst, say like ethanol won't make ethyl iodide just like that...
[Edited on 19-8-2009 by carbon chloride]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | | Perhaps then maybe add I2 to vinyl acetate and then like decarboxylate it...? |
It's an ester, decarboxylation isn't going to be easy and won't do what you want it to.
Instead of a simple ester or ether, like ethyl vinyl ether, use a group that will be fairly easy to remove after forming the vinyl and addition of
iodine.
Another possibly route would be to start with say 1,2-dibromo ethylene (why did you pick the iodide anyway?), made by adding Br2 to acetylene, and
adding water to it.
I think you'd have better luck starting with benzoin, then picking a sequnce to reduce the hydroxy or carbonyl group, then go to the amine.
Interesting neurotoxic properties.
However you're not going to easily get that compound to react with benzene, the hydroxy group is rather reactive to many of the reagents used to get
aromatic to couple to alkyl halides, although some of the Pd based chemistry might do it.
|
|
|
carbon chloride
Harmless

Posts: 12
Registered: 18-8-2009
Member Is Offline
Mood: No Mood
|
|
Right I didn't mean decarboxylation I meant something else... hmm yeah benzoin that's what vesp told me also. I didn't fully think over yet how that
would work...
Really, the hydroxy group is reactive? I thought iodine or bromine would be 20 times better at alkylating/methylating a benzene group... I don't even
know what to think any more.
Edit: after thinking about it yeah benzoin is 100 times better... either reduce that =O to H or that -OH to H and that =O to OH and I guess you would
have everything than this whole alkyl halide mess.. I'll have to think about how to synthesize benzoin... there's probably a bunch of patents.
[Edited on 19-8-2009 by carbon chloride]
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
*facepalm*
Am I seriously the only person who realizes that the desired molecule at the top is going to eject HI, forming 2-iodoacetaldehyde, which will then
undergo numerous condensations due to the acid catalysis?
This seems remarkably like spoonfeeding, although it toes the line.
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
carbon chloride
Harmless

Posts: 12
Registered: 18-8-2009
Member Is Offline
Mood: No Mood
|
|
Lol maybe it will, I didn't think about that...
In any case don't worry I've forgotten all about it, it seems like it's going to be so difficult to synthesize and benzoin now seems incredibly
easy...
[Edited on 19-8-2009 by carbon chloride]
|
|
|
kclo4
National Hazard
   
Posts: 916
Registered: 11-12-2004
Location:
Member Is Offline
Mood: No Mood
|
|
Yeah, if you have access to benzoin.
.. or the means to make it with a thiamine or cyanide catalyst and benzaldehyde.
I believe the best way is via the barbier reaction, but I won't talk about it as I've already said it at TV and against the rules of
sciencemadness...sorta
|
|
|
carbon chloride
Harmless

Posts: 12
Registered: 18-8-2009
Member Is Offline
Mood: No Mood
|
|
Yeah that's what I was thinking benzaldehyde with thiamine it's quite easy only requires ethanol and NaOH... And so this is vesp...
[Edited on 19-8-2009 by carbon chloride]
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
Quote: Originally posted by carbon chloride  |
Just a stupid idea I've had kclo4, you might have seen it on the vespiary (although I kind of ashamed myself over there don't know if I should go
back)...
|
You come over there make over 12 post per day then say your ashamed to be over there? Hmmmm, Im a bit offended and perhaps you shouldn't comeback
because where not about spoonfeeding ungreatful addicts!
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
carbon chloride
Harmless

Posts: 12
Registered: 18-8-2009
Member Is Offline
Mood: No Mood
|
|
no, you misunderstood me... what i meant was i ashamed myself over there by making stupid posts,
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
My apologies then.
It is just difficult to keep order in any forum that allows the discussion of mind altering or explosive substances and I have seen many two faced
that will visit one local yet publicly refute it when they come here. I believe that someones intrest are what they are. We are taking pride in not
allowing the forum to degrade into the common everyday meth tek while trying to make the place as user freindly as possible. There are many bright
minds over there and just would hate to hear someone say they are ashamed to be a member when they are.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
Rich_Insane
Hazard to Others
  
Posts: 371
Registered: 24-4-2009
Location: Portland, Oregon
Member Is Offline
Mood: alive
|
|
I think I've got it..... tell me if I've said anything wrong (I've only had the most basic of Organic Chemistry). Take ethan-1,2-diol (antifreeze).
Kick out those damn hydroxyl groups with KI (which is a good nucleophile). Do it twice instead of adding 2x at once. Then add a Br or Cl group with
HCl or HBr, take the product immerse in Conc NaOH, and there it is.
Funny how nucleophilic substitution is all I remember very well these days.
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
Did you read the part where U_C stated the product was unstable and will eject HI ending in a polymer goo more then likely.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
Rich_Insane
Hazard to Others
  
Posts: 371
Registered: 24-4-2009
Location: Portland, Oregon
Member Is Offline
Mood: alive
|
|
You mean the requested product?
I'm just suggesting a possible pathway to reach the product.
|
|
|