dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Bismuth Nitrate
Hello,
I want to make some Bismuth Nitrate from lab grade, 70% Nitric acid and Bismuth metal(from ebay as pure metal).
Do I just add some Bismuth metal to an excess of the acid and evaporate to get Bi(NO3)3:5H2O ?
TIA,
Dann2
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
That would be basically it. However, because it is an exothermic reaction, cooling of the reaction vessel would be necessary. Also, because HNO3 is an
oxidizing acid, the reaction will evolve not just H2 but also NO and NO2, the latter as a toxic intensely orange-brown vapor with a penetrating odor.
|
|
|
Fleaker
International Hazard
    
Posts: 1252
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
I don't remember the reaction of bismuth metal and nitric acid being all that vigorous. I think I took bismuth pellets and smashed them into a coarse
powder (bismuth is rather brittle). I think you may want to use less concentrated nitric acid, as I vaguely recall making just the oxide/hydroxide and
not much of the nitrate. I got the nitrate by dissolving it in dilute nitric acid.
Neither flask nor beaker.
"Kid, you don't even know just what you don't know. "
--The Dark Lord Sauron
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
Funny you should mention bismuth nitrate, I've just been using some to dope a new MnO2 anode for perchlorate.
When I made mine a while back, I just added pea size pieces of Bi into about 50 ml of 68% nitric acid in a 100 ml beaker until the reaction died away.
It was a fairly vigorous reaction with plenty of NO2 so do it outside. I left it overnight, outside, and in the morning the beaker was about a third
full of crystals. I never tried to dry the crystals, and have just left them moist with the remnant nitric acid, as I thought they might decompose
otherwise. They have kept perfectly well.
There is some information on bismuth nitrate solubility by Rosco and Xenoid on page 6 of the Multilayer Metal Oxide / Titanium Anodes for
Chlorate/Perchlorate thread!
Damn!! I had not realised it crystallised as a pentahydrate, I'll have to do my calculations again.
[Edited on 27-12-2009 by Xenoid]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
You might wish to download a copy of the following
http://www.archive.org/details/basicnitratesofb00allauoft
which tells you how to convert the nitrate to various basic nitrates, which you likely wish to avoid :-)
Also likely to be useful. A Text Book Of Inorganic Chemistry Volume VI Part V Antimony And Bismuth
http://www.archive.org/details/textbookofinorga028836mbp
And this. from Laboratory Methods of Inorganic Chemistry by Heinrich Biltz @ Google books, is the preparation of the true nitrate - I cut off
the portion to do with the basic nitrate. The HNO3 used, sg 1,2, is 33% or roughly half the standard concentrated acid.
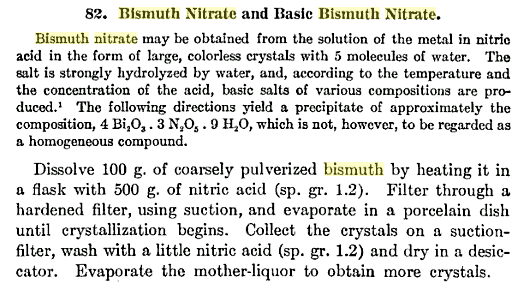
[Edited on 27-12-2009 by not_important]
|
|
|