Bedlasky
International Hazard
    
Posts: 1219
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Metal heteropolymolybdates
Hi.
I did few experiments with heteropolymolybdates in last days. Most of you probably read about heteropolymolybdates of metalloids and nonmetals - like
B, Si, Ge, P, As, Sb, Te etc.
But there are also metal heteropolymolybdates which aren't that often mentioned. They are formed in acidic solution (but they are destroyed by higher
concentration of acids, best resistivity to acids have VV heteropolymolybdate). I often prepared them by mixing of metal salt with excess
of ammonium heptamolybdate. This solution have optimal pH for formation of all metal heteropolymolybdates, so no acid is needed.
For VIV and CuICuII I used sodium metabisulfite as reductor.
Colours of solutions:
VIV: Very dark purple
VV: Orange to yellow-orange
CrIII: Pink in acidic/green in neutral solution
MnII: Yellow
FeII: Dark brown
FeIII: Colourless in acidic/brown in neutral solution
CoII: Red
NiII: Firstly pale green, than turquoise and finally blue (similar to CuSO4 solution). Complex formation is slow, it take some time (I let
it stand for a day).
CuICuII: Dark brown
CuII: Grennish blue
ZnII: Colourless
AlIII: Colourless
Ammonium salts of these heteropolymolybdates have quite low solubility in cold water (but in hot water are soluble - at least trivalent
heteropolymolybdates are, from divalent once I tried heat only Co, Ni and Mn). Co and Ni heteropolymolybdates are unstable in hot solution - after
heating you obtain precipitates of Co and Ni "molybdates".
FeII-polymolybdate is unstable and it decomposes in to molybdenum blue and ferric ions.
Ammonium salt of VV-polymolybdate is insoluble. If you add some NH4Cl and heat the solution, you obtain orange precipitate. Potassium and
rubidium salts are soluble - so there isn't similarity with phosphomolybdates.
Other ammonium salts slowly crystallized out. This is slow process - I must let it stand for a day, but two days will be better.
Colours of solids:
VIV: ? (I have probably too low concentration of V in solution, so there wasn't any crystal formation)
VV: Orange
CrIII: Pink
MnII: Yellow
FeIII: White
CoII: Beige
NiII: Light blue
CuICuII: Mix of light grey and dark brown
CuII: Pale grennish blue
ZnII: White
AlIII: White
If you add metal salt in excess in to the molybdate solution, you obtain "metal molybdate" precipitates (probably with V exception).
CrIII: Green
MnII: White (sometimes with very pale yellow tint)
FeII: Dark brown
FeIII: Brown
CoII: Violet
NiII: Pale green
CuICuII: Dark brown
CuII: Turquoise
ZnII: White
AlIII: White
I'll add later some pictures.
Btw. Formation of SnII-polymolybdate is responsible for quick reduction of molybdate by SnII.
Some literature: https://open.bu.edu/ds2/stream/?#/documents/223173/page/1
I tried preparation of CoIII-polymolybdate but without success.
[Edited on 29-3-2020 by Bedlasky]
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
Hi, That's a great overview of the colours these compound take. And a lot of work in testing all of this. Thanks for sharing.
I wonder, have you considered to add an oxidiser to the Co(II) and Ni(II) solutions? It seems that the higher oxidation state contributes to the
stability of the molybdate complexes, so that might work either way for stabilising Co(III) and Ni(III) or Ni(IV) polymolybdates.
I have tested Pr(III) and Er(III) with ammonium heptamolybdate, but nothing spectacular happened. I think they just precipitate the regular molybdates
Er2(MoO4)3 and Pr2(MoO4)3. It could be that these f-block elements are just a size to big for forming a heteropolymolybdate.
|
|
|
Bedlasky
International Hazard
    
Posts: 1219
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Thanks.
I tried to oxidized Co(II) and Mn(II) with persulfate on hot water bath, but without succes.
When I'll do experiments with cerium, I'll try reactions of Ce(III) and Ce(IV) with molybdate and tungstate.
I yesterday uploaded photos in to the computer. I'll upload it at evening on SM.
|
|
|
Bedlasky
International Hazard
    
Posts: 1219
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Here are promised pictures.

Cr(III)

Cr(III) on the left, Fe(III) on the right

Fe(III)

Al(III)

Mn(II)

Mn(II)

Fe(II)

Co(II)

Co(II)

Ni(II)

Ni(II)

Cu(I)Cu(II)
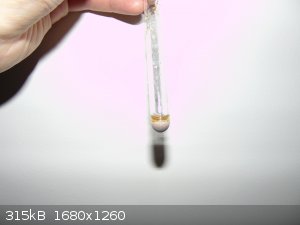
Cu(I)Cu(II)

Cu(II)

Cu(II)

Zn(II)

V(IV)

V(IV)
![Na3[VMo12O40].JPG - 375kB](https://www.sciencemadness.org/whisper/files.php?pid=633547&aid=80202)
V(V)
[Edited on 3-4-2020 by Bedlasky]
|
|
|