DennyDevHE77
Hazard to Others
  
Posts: 148
Registered: 15-9-2014
Member Is Offline
Mood: No Mood
|
|
Picric acid via sulfanilic acid
[I knew I wouldn't get an answer in the profile topic (Picric acid: different instructions) So I'm gonna move it over here]
Hello everyone, there is a recipe for the preparation of picric acid from sulphanilic acid without the use of concentrated sulphuric acid.
If anyone is not aware, I will give the methodology written out of the book "Laboratory preparation of explosives" by Solonin A.A..
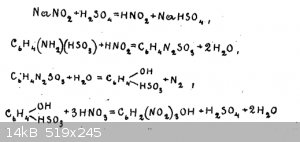
"30g of sulfanilic acid is shaken with 250 cm³ of water and with 12g of sodium salt of nitrous acid. After careful stirring, carefully under strong
cooling with ice water, about 100 cm³ of the cooled solution of 10 grams of sulphuric acid of specific gravity 1.84 g/cm³ in 100 cm³ of water is
poured in.
After adding the sulphuric acid while cooling, the mixture is stirred from time to time for one or two hours, and left to stand for two hours at
ordinary temperature. The obtained porous mass is filtered on a Buechner funnel, transferred into a beaker and then, with stirring and heating on a
water bath poured 96g of nitric acid of specific gravity 1.38 g/cm³.
At the end of pouring nitric acid, heating is carried out until the end of gas emission and then left to stand for a day.
The released picric acid is filtered off on a Buechner funnel, washed with cold water."
There is a scheme of reactions (on the picture)
I was attracted to this method because sulfanilic acid costs about the same as phenol, and in general all substances are legal and easily available.
Also, I'm currently experiencing some problems with concentrated sulfuric acid. But I still haven't found correct information on the yield. And also
the exact temperatures are not specified, and I think there may be pitfalls here.
If someone has experience in synthesis of picric acid by this method, please share your experience, thanks in advance. Personally, in general, this
method seems to me to work, but I do not expect large yields, as the molecule of sulfanilic acid is already heavy enough.
|
|
|
DennyDevHE77
Hazard to Others
  
Posts: 148
Registered: 15-9-2014
Member Is Offline
Mood: No Mood
|
|
Practice
So, after getting all the reagents I needed, I decided to give it a try.
First of all, having mixed 30 g of sulfanilic acid and 12 g of sodium nitrite in a common cup, I poured them into 250 ml of water (cold), after a
short stirring the mixture almost immediately became carrot-colored.

Having mixed 10g of 95% sulfuric acid and 100g of water in a separate cup, I quietly started pouring them into the mixture lowered into the ice bath.
I expected a strong temperature rise, but in fact the temperature rose by 4-5°C. After a few minutes the mixture turned a pale yellow color

Following the instructions, I held the mixture for 2 hours while refrigerated (stirring occasionally), and another 2 hours at room temperature. As a
result, the yellow precipitate settled to the bottom and the bright yellow solution remained on the surface. The yellow precipitate was filtered off.

The squeezed yellow mass was then added to 90g of 70% nitric acid. At first it floated in lumps, but soon dissolved

I didn't know exactly how hot the mixture should be heated to, but I found a similar synthesis technique in a book.
 
Based on it, it should be heated to 65-70°C and then brought to a boil. At heating up to 55°C the first signs of reaction began, bubbles started to
come out in the solution, when exceeding 75°C the mixture started to boil. I tried to keep the temperature at 68°C. Small amounts of NO2 were also
coming out.
After keeping it for about 3 hours, the gas emission decreased and the mixture darkened.
Due to the difference in methods, it was decided to pour part of the solution into a separate container and keep it for 24 hours, and part of it was
boiled at 110-115°C.

Nevertheless, the end result, in both cases, was clearly not picric acid, but a dark red crystalline substance. I do not know what it is, I almost did
not touch it, it turned out only that it is perfectly soluble in water, melts from a burning match, but does not burn and does not support fire.

I don't know, maybe I followed the wrong methodology, or maybe they are taken from non-working patents in general.... But if anyone can guess what
kind of reactions were going on here, and what kind of product was obtained in the end, I'd be glad to hear your ideas.
|
|
|
DennyDevHE77
Hazard to Others
  
Posts: 148
Registered: 15-9-2014
Member Is Offline
Mood: No Mood
|
|
Correction, after I decided to wash this red precipitate, this red substance dissolved almost immediately, exposing picric acid, which I
recrystallized afterwards from boiling water (not very qualitatively).
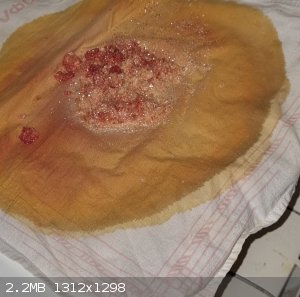 
However, for 30g of sulfanilic acid I got 15g of dirty picric acid, and 10g after recrystallization. Anyway, I'm not impressed.
I think that sulfanilic acid should be used only as a light source of monosulfophenol. It can be nitrated in the old industrial way with dilute nitric
acid and nitrates.
In general, I'm becoming more and more disillusioned with nitroaromatics. Everything from this area is nitrated for a long time, at high temperatures,
and eats a lot of sulfuric acid. In my opinion, the only advantage is that many compounds can be cast and high density can be achieved.
|
|
|
|