Hey Buddy
Hazard to Others
  
Posts: 386
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
NENO
Dinitro Dioxyehtyl Oxamide Dinitrate. Found this reference in PATR. GerP 530,704 (1930). Aminoethanol Acyl derivative, condensation of Oxalic Acid
with MEA. Searched a little more, found the last two screen shots. Density 1.72 OB -18% CO2 mp 88 C. MEA dinitrate alone is supposedly 93% TNT on
trauzl with 410cc with a density of 1.53g/cc.
I worked on the condensation, I believe it works best dissolving oxalic acid in ethanol, then dripping in MEA with stirring. Addition is exothermic.
You can use water instead of alcohol but its harder to dry. Alcohol dries much faster. Also yields with IPA. Finished product is a sparkly white
powder. Yield is 80% of theoretical. So far, everything looks good. Tried nitration with 96%NA for several hours. Goes through radical changes in
color white/orange/red/then white again. No precipitation on crashing, you can see oily substance in crash.
Attempted sulfuric acid nitrate salt mixed acid, same result. Finished product should be insoluble if source info is correct. What would be the
logical next step to attempt to pull off a finished yield? Is it possible the condensed product is a salt, rather than the oxamide desired? Not really
sure. Just been making TNT but would love to find something a little denser more OB and powerful.

 
|
|
|
Microtek
National Hazard
   
Posts: 828
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
This is the same material I posted a thread about some time ago:
http://www.sciencemadness.org/talk/viewthread.php?tid=159807
Klapötke has done some work on it and I attached the paper in that thread. I haven't had the time to do further experiments with the nitration yet,
but one difference in the preparation of the substrate is that Klapötke reacts ethanolamine with diethyloxalate rather than directly with oxalic
acid. I would think that you would get a salt instead of a condensation product otherwise.
[Edited on 3-11-2023 by Microtek]
|
|
|
dettoo456
Hazard to Others
  
Posts: 178
Registered: 12-9-2021
Member Is Offline
|
|
You could try to condense ethylenedinitramine with monoethanolamine and then nitrate fully to the 1,4,7,10-tetranitrazadecane (worse OB% derivative).
That would get rid of the ketones which could be destabilizing towards the a-nitro groups on the amide. I don’t know how stable nitroamides are
though.
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 386
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
Quote: Originally posted by Microtek  | This is the same material I posted a thread about some time ago:
http://www.sciencemadness.org/talk/viewthread.php?tid=159807
Klapötke has done some work on it and I attached the paper in that thread. I haven't had the time to do further experiments with the nitration yet,
but one difference in the preparation of the substrate is that Klapötke reacts ethanolamine with diethyloxalate rather than directly with oxalic
acid. I would think that you would get a salt instead of a condensation product otherwise.
[Edited on 3-11-2023 by Microtek] |
I hadnt even realized these were the same.
From the old patents, it seems likely they are forming the oxamide with nothing but MEA/oxalic, I suppose high temp or some sulfuric acid addition
maybe? There isnt a mention of further processing. I do imagine that what is had from reaction in alcohol is a salt. Oxalic and MEA out of alcohol
react really hot, releasing a lot of gas seems like mostly CO2 maybe CO. Basically uncontrollable gas release and decomposition. Alcohol really calms
it down to a slow hissing on MEA addition and a thick paste forms. I suppose I should attempt to heat it past 100 C to determine if that affects a
condensation.
It would be ideal if NENO could be made in only two steps. Otherwise it seems like TNT would remain more expedient.
I get the oxalic and MEA from alliance chemical here in USA. They also have a lot of other solvents ethyl acetate for k6 etc. (If anyone in USA is
wondering where to get MEA)
[Edited on 3-11-2023 by Hey Buddy]
|
|
|
Microtek
National Hazard
   
Posts: 828
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
Diethyloxalate is easy to make from ethanol and oxalic acid. It is a high boiling point liquid that is quite insoluble in water. I make it by
refluxing a mix of the two, and then distilling ethanol and water off at room temp before applying vacuum to distil the diethyloxalate. You can boost
the yield by using molecular sieves in a Soxhlet setup to drive the equilibrium, but it isn't necessary to do so. The reaction between diethyloxalate
and ethanolamine is smooth and goes to completion as the byproduct ethanol evaporates.
TNT may be more expedient, and is certainly more fully described in litterature, but NENO is more powerful.
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 386
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
I would love to figure NENO out. Not sure what next step is... i guess heating the ox amide? I got kind of burnt out on attempting it after wasting so
much nitric acid on failures, and crashing it into water with no precipitate...
I turned attention to TNT. Got a pretty good procedure worked out with drain cleaner/AN. It's a one pot based off the old clint book TNT one pot
industrial procedure. Uses a lot of drain cleaner though. But it yields quite a bit of TNT with no DNT intermediate stage. Pretty high mp ~75 C+ even
without sulphite extraction. After sulphite it should clean the mp up pretty good removing all those isomers and MNT.
|
|
|
Sir_Gawain
Hazard to Others
  
Posts: 272
Registered: 12-10-2022
Location: Due South of Due West
Member Is Offline
Mood: Yes.
|
|
Please share your TNT procedure!
“Alchemy is trying to turn things yellow; chemistry is trying to avoid things turning yellow.” -Tom deP.
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 386
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
Sure.
Procedure is from G. Carlton Smith, not "clint", lol. I have been using his cited TNT single stage method from the pdf (page 33) here on SM. I
modified it for use of AN and 90%+ drain cleaner. No water is added. Water is produced as the reaction proceeds.
75% Sulfuric Acid w/w (do account for lower concentration drain cleaner, if used)
25% Nitric acid w/w (supplementing NH4NO3)
One part toluene for 12 parts mixed acid w/w
1 g Toluene / 9 g H2SO4 / 3 g HNO3
1 g Toluene / 15 g Drain cleaner / 3.76g NH4NO3 (or 3.99 g NaNO3)
NaNO3 has a different mass concentration and viscosity, this example is NH4NO3.
From the photos below, a mixed acid of 810 ml drain cleaner / 376 g NH4NO3 was prepared.
Drain cleaner used had no MSDS and was unknown concentration and had a purple dye, the acid was about the color of a "purple drank" ; ). No
purification of draincleaner needed.
(85% and below sulfuric acid will freeze in about two days, this drain cleaner was 90 % + but density wasn't measured.)
It was planned to drop 100 g toluene into that mix but it was slightly too great a volume for a one liter vessel.
10% of the toluene and 10% of the acid by weight were subtracted.
In 1000 cc space, it was possible to nitrate 90 g toluene, but it's cutting it close.
In pictures you can see 3 neck flask is filled to the necks with no head room, which is really too much fill and not ideal, but doable. Sulfuric acid
expands in volume during heating. In the photos below you can see a comparison of the volumes: the grapefruit color is acid mix volume at ~45 C, the
orange juice color is mixed acid volume at 120 C.
Drip in toluene into mixed acid and attempt to keep it under 30 C (not likely, I usually exotherm to somewhere around 45-50C with a slow drip).
When all the toluene is dripped in and exotherm is complete, temp drops.
Color is "grapefruit juice" at this point, this is MNT.
Change out dropping funnel for a condenser.
Temp is raised to 90-95 C for 2 hours.
Color change: pink to orange, bubbling.
After 2 hours at 90 C, temp is raised to 120 C for 2 hours.
More bubbling and off gassing occurs.
Color change: "orange juice".
After the 2 hours nitration period at 120 C, nitration is complete
Dinitro is made at 90 C trinitro at 120 C
Pour out highly acidic mix into water to precipitate crude TNT. Then begin processing purification. 80.2 C mp is the target of purification. Not sure
on yield for AN / draincleaner method, because im waiting on shipment of sulfite.
Carlton Smith claims yield is 1.9 g TNT per g toluene.
I had to use overhead stirrer. I dont think it possible to do it with magnetic stirrer.
Produced fumes are too great for open vessel, requires enclosed vessel with tall condenser. Or do it outside with loss. Heating too much during
toluene addition promotes oxidation. Oxidation creates unwanted products like tetranitromethane, but if this is produced, it is removed in
purification.
First pic is crude TNT at completion after diluting with equal volume of water.
I decant this waste liquor, then hot wash with 70% H2SO4 @ 75 C+, then dilute with water and cool, which forces it into solidification from molten
droplets, then decant. Mass is then dissolved in hot 91% IPA from the dollar store. just enough to dissolve everything with heat, this is where TNT
will fully dissolve into a dark red alcohol solution. It is stirred a few minutes in dissolved state, then diluted with water, opaque yellow liquid
results, TNT solidifies at bottom. Once cool, decant. Repeat this alcohol recrystallization many times. IPA volume only needs to be enough to dissolve
TNT. Each time the TNT gets more tan / light colored and less yellow / red. As it is purified, it becomes less amorphous and begins crystallizing in
needles. The mp also goes up. Crystals begin on the top of the crude TNT and work their way down until it is purified. I suspect sulfite wash buffered
to pH 7 with boric acid is superior to alcohol wash in time and labor, but alcohol wash with IPA is available OTC, while sulfite must be sourced.
Overall, TNT is simple with a little equipment. Purification is pretty messy, it produces a LOT of waste liquor. However, if TNT is needed, IMO single
stage with nitrate salt/draincleaner is better than 2 stage or 3 stage, as you dont have to process with as much time and labor. Simply regulate temp.
  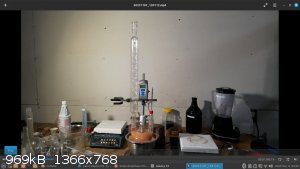  
[Edited on 8-11-2023 by Hey Buddy]

[Edited on 8-11-2023 by Hey Buddy]
|
|
|