DennyDevHE77
Hazard to Others
  
Posts: 148
Registered: 15-9-2014
Member Is Offline
Mood: No Mood
|
|
Drying of nitric acid with magnesium nitrate
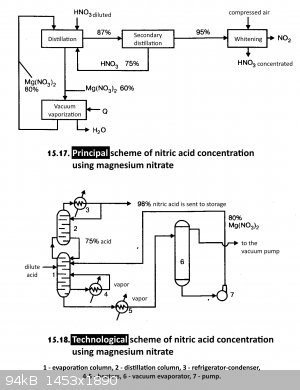
Found this method of drying nitric acid. Below is a description and both diagrams.
"Dilute nitric acid is introduced into evaporation column 1 and an 80% solution of magnesium nitrate is fed from Vacuum Evaporator 6. The 97%
nitric acid vapor leaving the evaporation column flows into distillation column 2 and from there through refrigerator-condenser 3 into storage. The
75% nitric acid released from the bottom of the distillation column is returned to the evaporation column. Diluted to a concentration of 50-60%
magnesium nitrate solution from the lower part of the evaporation column is directed to the vacuum evaporator 6 and from there, after evaporation to
the initial concentration (80%) is pumped by pump 7 to the evaporation column."
The question is actually simple, can this industrial setup be replicated as a laboratory dish assembly? Or at least reduce it and make a miniature
version? Just magnesium nitrate is very cheap, and here is not even anhydrous salt but 80% solution.
I've tried distilling with dihydrate. I only got 75% acid. I think I need to use a deflegmator, but I could be wrong. Is a deflegmator analogous to an
evaporation column?
|
|
|
Sir_Gawain
Hazard to Others
  
Posts: 272
Registered: 12-10-2022
Location: Due South of Due West
Member Is Offline
Mood: Yes.
|
|
Nurd Rage made a video about this IIRC.
“Alchemy is trying to turn things yellow; chemistry is trying to avoid things turning yellow.” -Tom deP.
|
|
|
Sir_Gawain
Hazard to Others
  
Posts: 272
Registered: 12-10-2022
Location: Due South of Due West
Member Is Offline
Mood: Yes.
|
|
Here it is: https://youtu.be/88gbfCnrV8o?si=oZAsuG4IdCddzIHP
“Alchemy is trying to turn things yellow; chemistry is trying to avoid things turning yellow.” -Tom deP.
|
|
|
Rainwater
National Hazard
   
Posts: 800
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
When i here dephlegmator, i get thirsty
To my knowledge that is a non-adiabatic fractionating column. Lots of fancy words for something simple. It is supose to lose heat to help reflux the
distilate. It is the equivalent of a youtube fractional distillation.
I don't see how that could work as drawn. Without a dehydrating agent in the second distillation the distillate would not be further enriched.
Lets assume the second distillation also has a flow of dehydrating agent.
In a bulk process, you would want to keep the first boiling flask at a tempature above 120c to ensure the removal of your product. Once thats done,
move a portion of the solution for drying/consentration
Then dump the consentrated dehydrating agent back into the second boiling flask, letting the overflow drop into the first and repeat.
So the only real problem is how to transfer hot magnesium nitrate solution contaminated with nitric acid. You picked a tuff one
"You can't do that" - challenge accepted
|
|
|
DennyDevHE77
Hazard to Others
  
Posts: 148
Registered: 15-9-2014
Member Is Offline
Mood: No Mood
|
|
There it is indicated from above (with an arrow) that part of 98% of nitric acid is drained back, that is, as I understand it, it goes as a phlegm.
Won't that increase concentration?
|
|
|