palico
Hazard to Self
 
Posts: 70
Registered: 1-10-2013
Member Is Offline
Mood: No Mood
|
|
Preparation of biodiesel from waste cooking oil
Hello people,
today we an experiment which I am very proud of, and it is also the best video on my YT channel at this moment: the preparation of biodiesel from waste cooking oil.
The reaction involved is the transesterification of oil with ethanol using sulfuric acid as catalyst.

This experiment present different point of novelty compared to common biodiesel production method. First, it use waste cooking oil as substrate, and
so has the advantage to convert a waste into a resource. Second, ethanol is used rather than methanol, which the purchase in some countries can be
restricted for individual or hardly to find. Ethanol instead, is free to buy everywhere, readily available, cheap, and also can be renewable, because
obtained from wine and spirit industry. Third, I am using an acid recipe, not basic, which is not sensitive to residual water, moisture, free fatty
acids.
No complicated oil pre-titration is needed, the reaction is then more versatile and can accept various pil charge.
Procedure
The oil is pre-treated as follows: oil is collected, filtered roughly through a steel strainer, and the again through a tighter one. The oil is then
heated to 100 Celsius on hotplate under stirring and kept for hours, until all water and volatiles are boiled off.
Once cool, 100 g of waste cooking oil is introduced in a 250 ml round bottomed flask, then 40 ml of 90 degree denaturated ethanol are added. The
reaction mixture is biphasic. 1 ml of concentrated sulfuric acid is pipetted in, the reaction mixture brought to 90 Celsius in oil bath, and gently
refluxed at this temperature for 8 hours. Initially, reaction mixture becomes soapy and single phase. Bubbles also decrease to disappears in few
hours, evidence of ethanol getting consumed and transesterification going on. The reaction mixture becomes also more limpid.
Once finished the flask is removed from bath, let cool down to room temperature: two phase are seen, which the bottom is glycerol. Glycerol is removed
in separatory funnel, the biodiesel washed with 3 x 50 ml water. A strong emulsion forms, indicating still presence of mono and di-glycerides.
A last washing with 5% bicarbonate solution is done.
The product is opalescent, is filtered through cotton plug, then heated under stirring at 80 Celsius to remove all volatiles: product become limpid
and clear.
An yield 68 g, 76 ml of yellow oily liquid is obtained.

Discussion
Product is characterized by density, refractometry, flame test and compared to initial waste oil. Results are the following.
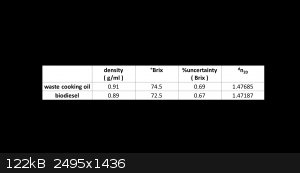
It is shown, product has lower density and refractive index, and this is as expected for a shorter chain molecule. The flame test is done as follow. A
paper towel is impregnated with the sample, placed in a steel can and ignited. The oil is diffcult to catch on fire. Its combustion is awful. It
generates acrid, toxic terribles fumes. The biodiesel instead, catch easily on fire, the combustion is smooth and stable and does not generates
terrible fumes.
The behaviour of both on freezing is identical.
I am planning to repeat this reaction with more ethanol, and then with 96 degrees denaturated ethanol.
As usual I thank you for attention and link to YT video for a more detailed procedure.
See you,
palico
|
|
|
Dr.Bob
International Hazard
    
Posts: 2913
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: Mildly disgruntled scientist
|
|
That is a great experimental. I have made methanol based biodiesel before, and know that ethanol is harder to make it with, so that is a good job.
And not only is biodiesel less toxic, it is actually drinkable, used as a treatment for heart disease.
Good luck with work, I am happy that it is working. Even better if someone could find a biochemical method for producing ethyl biodiesel, or
something as safe as that, and lower the energy needed to make it. That could make a safe fuel even more practical. It would also be great to
bioengineer plants to make the oils better in the US climate, since we cannot get the high yields of palms in the tropics with soybeans or rapeseed
crops. Thus people are clearing millions of acres in tropical rain forests.
|
|
|
palico
Hazard to Self
 
Posts: 70
Registered: 1-10-2013
Member Is Offline
Mood: No Mood
|
|
Hello Dr. Bob. To know if that biodiesel is good for trasportation it requires more accurate analysis and fit law-based requirements as cetane number,
metal impurities level, but I think instead it is good enough as heating fuel or for power generator, since waste cooking oil cannot be used directly
for any combustion. In EU doing so it is envoironmental crime. The emulsion is evidence of mono and di-glycerides presence, but it can be mixed with
fossil diesel in case.
Thanks for comment.
|
|
|
Texium
|
Thread Moved
29-3-2024 at 12:42 |
|