Niklas
Harmless

Posts: 27
Registered: 1-12-2023
Location: Germany
Member Is Offline
Mood: Polymerized
|
|
Failed attempt on making glycouril
Hey everyone, I recently found out about cucurbiturils, and their surprisingly simple two-step synthesis starting from the common laboratory chemicals
urea, glyoxal and paraformaldehyde:
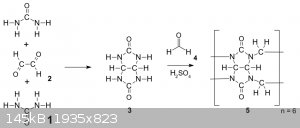
Due to me already having all the required around I randomly decided to just go for it, attempting the first step today according to the following
procedure found in http://dx.doi.org/10.1080/17518253.2012.718803 :
“To a water (5 mL) solution of 1,2-dicarbonyl compound 1 (3.0 mmol), P4O10 (1.5 mmol, 426 mg) was added and the mixture was stirred for 5 minutes.
After this time urea 2 (9.0 mmol, 540 mg) was added. After 10 minutes the solution became cloudy and the product precipitates. The product was
collected by filtration on a Buckner funnel and the solid was washed with cold water and dried. The products were characterized by 'H and 13C NMR
spectroscopy.“
I did scale up the procedure by a factor of 33, and I did use a little more water than intended since glyoxal is only available as a 40% aqueous
solution, other than that I exactly followed things as shown above.
And things seemed to be working quite well at first, with the solution turning slightly cloudy shortly after the urea was added, to my surprise things
went clear again a couple of minutes later tho with a product refusing to crystallize out even after scraping the flask and cooling down the reaction
mix..
As a last attempt of getting something to crash out I brought the highly acidic solution to a neutral pH using sodium hydroxide solution, this only
caused things to quickly turn yellow, later black, destroying my last hope of the synthesis having worked..
    
And well, now I‘m pretty confused about things refusing to work, with this being an incredibly simple procedure found in multiple papers all
claiming to have gotten good yields.
My main suspicion is the glyoxal solution I got from a chemical supplier a while back somehow having gotten bad, I‘m not entirely sure how I could
verify this tho, and if there is really a chance of there being absolutely no glyoxal in the solution anymore.
All help and suggestions would be greatly appreciated, thanks in advance!
|
|
|
bnull
Hazard to Others
  
Posts: 167
Registered: 15-1-2024
Location: Between the Atlantic and the Pacific Ocean
Member Is Offline
Mood: Sleepy (again)
|
|
Correct me if I am wrong. You used 11.7 mL (14.5 g) of glyoxal 40%, 14.0 g of P4O10, 17.8 g of urea, and 156.3 mL of water (give
or take 10 mL).
The glyoxal may have polymerized. Take a couple of mL of glyoxal and put in the fridge. The polymers should precipitate in the cold if there are any.
Try it again in the scale given in the paper.
Quod scripsi, scripsi.
B. N. Ull
P.S.: Did you know that we have a Library?
|
|
|
Niklas
Harmless

Posts: 27
Registered: 1-12-2023
Location: Germany
Member Is Offline
Mood: Polymerized
|
|
Quote: Originally posted by bnull  | | Correct me if I am wrong. You used 11.7 mL (14.5 g) of glyoxal 40%, 14.0 g of P4O10, 17.8 g of urea, and 156.3 mL of water (give
or take 10 mL). |
That’s approximately the quantities I used, yeah.
Seems like there really is a whole lot of stuff precipitating out of the glyoxal when cooled down, left the bottle in my lab at around 8 degrees for a
few hours, and now there is a 1-2 cm layer of solid at the bottom..
Well, that sucks, looking through some SDS that seems to be a common occurrence though. I guess it’s best if I just freshly prepare my glyoxal from
ethylene glycol whenever I need it, got way too much of it anyway, so..
|
|
|
bnull
Hazard to Others
  
Posts: 167
Registered: 15-1-2024
Location: Between the Atlantic and the Pacific Ocean
Member Is Offline
Mood: Sleepy (again)
|
|
Quote: Originally posted by Niklas  | | Seems like there really is a whole lot of stuff precipitating out of the glyoxal when cooled down, left the bottle in my lab at around 8 degrees for a
few hours, and now there is a 1-2 cm layer of solid at the bottom.. |

Try this: transfer the solid stuff with some liquid to a beaker, add a couple of drops of sulfuric acid and leave it alone for a while. It works with
paraformaldehyde; with a little luck it may work with the glyoxal polymers.
Quod scripsi, scripsi.
B. N. Ull
P.S.: Did you know that we have a Library?
|
|
|
Niklas
Harmless

Posts: 27
Registered: 1-12-2023
Location: Germany
Member Is Offline
Mood: Polymerized
|
|
Quote: Originally posted by bnull  | | Try this: transfer the solid stuff with some liquid to a beaker, add a couple of drops of sulfuric acid and leave it alone for a while. It works with
paraformaldehyde; with a little luck it may work with the glyoxal polymers. |
It is pretty slow, but it works well enough apparently (what makes sense since it’s basically a bunch of paraldehdye-rings connected together
creating a thermoset polymer).
This really only confuses me even more tho, if depolymerization worked with such a small amount of acid present, how didn’t it just make glyoxal
with all the phosphoric acid in the reaction mix.? By the time I was setting up the reaction the polymers stills seemed to mostly be dissolved, so
they surely were added to the flask..
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2701
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
| Quote: | | I just freshly prepare my glyoxal from ethylene glycol whenever I need it, |
Could you expand on this?
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
Niklas
Harmless

Posts: 27
Registered: 1-12-2023
Location: Germany
Member Is Offline
Mood: Polymerized
|
|
I‘m not sure which method exactly I‘d use for making the glyoxal myself, making it from glycol seems like the most obvious method many glycoluril
papers actually mentioned for a preparation of glyoxal tho. At first I was confused by them not just buying the solution, since they were diluting
things down to 40% anyway, but well, I guess now we know why..
Obviously I wouldn’t make it via the silver catalyzed gas-phase oxidation with molecular oxygen, wouldn’t have the equipment for that
(unfortunately), but I believe I had seen some pretty otc preparations in a patent before.
Other than that oxidizing acetaldehyde with nitric acid seems like a feasible option, I just really don’t like working with ethanal tho, why even
disregarding the fact that I have 16 times as much lab grade glycol as I have acetaldehyde I‘d still prefer to use ethylene glycol as the precursor.
|
|
|
bnull
Hazard to Others
  
Posts: 167
Registered: 15-1-2024
Location: Between the Atlantic and the Pacific Ocean
Member Is Offline
Mood: Sleepy (again)
|
|
Quote: Originally posted by Niklas  | | It is pretty slow, but it works well enough apparently (what makes sense since it’s basically a bunch of paraldehdye-rings connected together
creating a thermoset polymer). |
I'm glad to know it worked. Maybe phosphoric acid acts as a stabilizer, or the urea sort of protects the polymers.
Quod scripsi, scripsi.
B. N. Ull
P.S.: Did you know that we have a Library?
|
|
|