CHRIS25
National Hazard
   
Posts: 951
Registered: 6-4-2012
Location: Ireland
Member Is Offline
Mood: No Mood
|
|
General query about 'assigned' chemical formulas
Hallo,
Just trying to learn here. I have noticed sometimes that a chemical aquires two different formulas. For example, sodium silicate is Na2Sio3. But I
have often seen this formula as Na2O3Si (Sodium metasilicate). Now I am presuming, by deduction, that when this happens it appears to be that the
Compound/chemical in question can not exist independently as Na2SiO3 and when in solution aquires a different formula. For example: NaOh + SiO2 =
Na2SiO3 + H2O.
Have I misunderstood what I am trying to understand?
Thanks
‘Calcination… is such a Separation of Bodies by Fire, as makes ‘em easily reducible into Powder; and for that reason ‘tis call’d by some
Chymical Pulverization.’ (John Friend, Chymical Lectures London, 1712)
Right is right, even if everyone is against it, and wrong is wrong, even if everyone is for it. (William Penn 1644-1718)
The very nature of Random, Chance development precludes the existence of Order - strange that our organic and inorganic world is so well defined by
precision and law. (me)
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
Some chemicals are unstable in aqueous solution, for example when sodium metasilicate is dissolved IIRC it is converted to sodium silicate.
Sometimes, the rearrangement of the atoms shown in formulae has to do with isomerism and what the reference that the user requires, e.g. the usual way
sodium hypobromite is written is NaOBr, but in the classic joke about it it is changed to 'NaBrO' for that purpose.
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
adamsium
Hazard to Others
  
Posts: 180
Registered: 9-4-2012
Location: \ƚooɿ\
Member Is Offline
Mood: uprooting
|
|
Sometimes, chemical formulae are written to emphasise the structure of a molecule, other times they are simply written in a way that lists how many
atoms of each element makes up a molecule (or at least the smallest unit - sodium silicate is actually an example of this) of that compound. A simple
demonstration of how this works can be made with ethanoic acid (or, acetic acid, as it is commonly known). The 'simplest' way of writing this would be
C2H4O2 (although, in practice, it is never actually written this way), as a molecule of acetic acid contains 2 carbon
atoms, 4 hydrogen atoms and 2 oxygen atoms. In practice, the molecular formula for acetic acid is written as either CH3CO2H or
CH3COOH. The first of these - CH3CO2H - indicates the molecular arrangement / structure to some extent, while the
latter - CH3COOH - elucidates the structure of the molecule most clearly of any of these as it implies the carboxylic acid (i.e. -COOH or,
better, -C(=O)OH ) group at the end of the molecule. These are all examples of condensed formulae.
The picture below shows a more complete structural formula. If one needed to show the 3 dimensional structure (i.e. the stereochemistry - very
important in organic chemistry, in particular), this structure would be drawn with the regular lines between the atoms replaced by hashed wedges to
indicate a bond going 'in to the page' and solid wedges to indicate bonds coming 'out of the page' where appropriate. This doesn't change the
condensed formulae you asked about, however, and I mention it only for the sake of completeness.
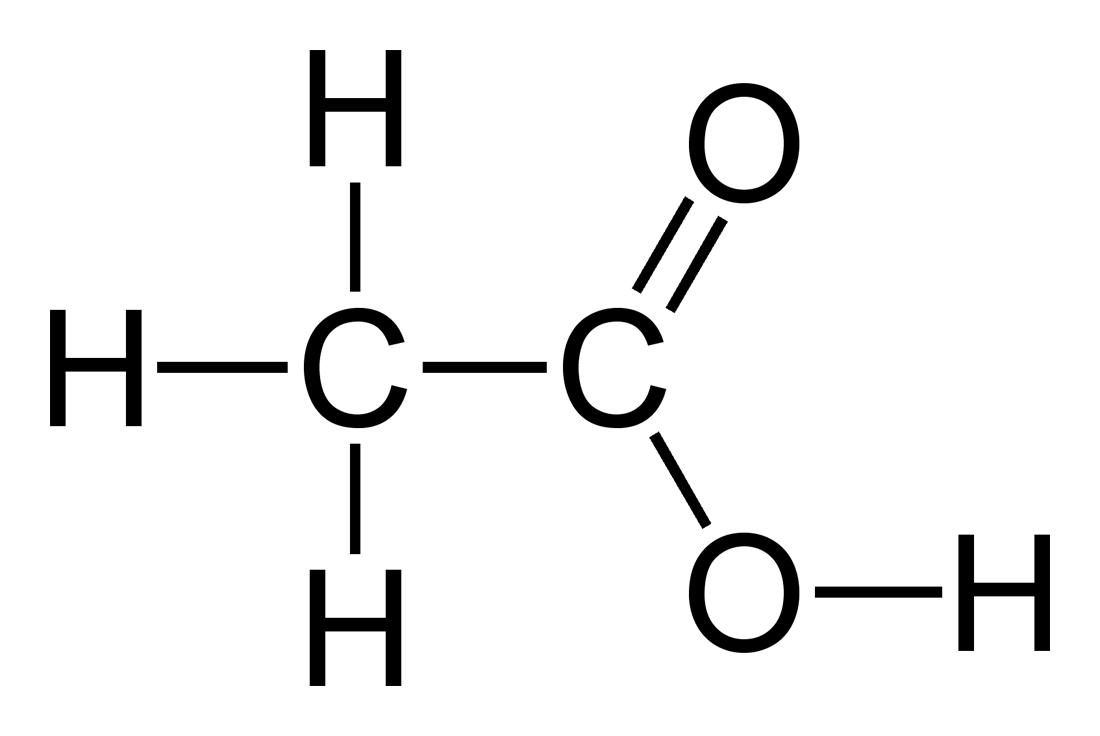
(Source: http://en.wikipedia.org/wiki/Acetic_acid )
As for sodium silicate, the silicate anion does not exist as a discrete anion, but is instead a polymeric anion. It can exist in both anhydrous and
hydrated forms and can be in solid crystalline form, or in a solution. Water of hydration is commonly indicated by appending "・nH2O
" or "(H2O)n" to a molecular formula, depending on the structure, where n is an integer indicating the number of water molecules
per molecule / unit of compound.
I hope this is helpful.
|
|
|
CHRIS25
National Hazard
   
Posts: 951
Registered: 6-4-2012
Location: Ireland
Member Is Offline
Mood: No Mood
|
|
Hexavalent, adamsium, yes most helpful, actually very elucidating - a number of terms for me to look up but that does not confuse me thankfully. I
had actually noticed many times the changing formulas used in ethanoic acid/acetic acid (why the two names I do not know). So this explanation really
clarifies things.
So preparations for melting some glass are underway, so much simpler than all the other reactions I had seen. Plus I can do it in a stainless steel
pan while waiting for my glass to come.
Kind regards
Sorry, what is iiiRC?
[Edited on 1-5-2012 by CHRIS25]
‘Calcination… is such a Separation of Bodies by Fire, as makes ‘em easily reducible into Powder; and for that reason ‘tis call’d by some
Chymical Pulverization.’ (John Friend, Chymical Lectures London, 1712)
Right is right, even if everyone is against it, and wrong is wrong, even if everyone is for it. (William Penn 1644-1718)
The very nature of Random, Chance development precludes the existence of Order - strange that our organic and inorganic world is so well defined by
precision and law. (me)
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
IIRC=If I recall correctly
Ethanoic acid is the systematic IUPAC name for this particular carboxylic acid, as it uses the standard 'eth' to represent x2 carbons. Acetic acid is
just a common/archaic name, in a similar fashion to methanoic acid/formic acid.
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
CHRIS25
National Hazard
   
Posts: 951
Registered: 6-4-2012
Location: Ireland
Member Is Offline
Mood: No Mood
|
|
Right, thankyou
‘Calcination… is such a Separation of Bodies by Fire, as makes ‘em easily reducible into Powder; and for that reason ‘tis call’d by some
Chymical Pulverization.’ (John Friend, Chymical Lectures London, 1712)
Right is right, even if everyone is against it, and wrong is wrong, even if everyone is for it. (William Penn 1644-1718)
The very nature of Random, Chance development precludes the existence of Order - strange that our organic and inorganic world is so well defined by
precision and law. (me)
|
|
|
|