Flip
Hazard to Others
  
Posts: 116
Registered: 7-12-2005
Member Is Offline
Mood: No Mood
|
|
Preparation of HgSO4
Does anyone know a viable method of HgSO4 preparation?
Does anyone know of any OTC sources?
I am interested in the conversion of terminal propynes to 2-propanones. Thanks guys.
Flip
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
HgSO4 can be prepared by either heating concentrated H2SO4 with mercury (don't know how the product is isolated here) or by dissolving HgO in dilute
sulfuric acid (better method).
HgO is prepared by dissolving mercury in a large excess of hot concentrated HNO3 and precipitating by adding NaOH solution to the diluted reaction
mix. HgO is filtered, washed and dried.
Dissolving HgO in a stochiometric amount of dilute H2SO4 then directly gives a pure solution of HgSO4.
If you could tell us more about your reaction scheme of the production of 2-propanones it would be nice, since it sounds interesting.
|
|
|
Flip
Hazard to Others
  
Posts: 116
Registered: 7-12-2005
Member Is Offline
Mood: No Mood
|
|
Synthesis of methyl ketones from terminal alkynes
Thanks for your response garage chemist... I wish that I had a good source for HNO3, because I have reservations about ordering it. I kinda figured
it would be along the lines of H2SO4 and heat but that would be a bit messy and I had hoped there was a better way. Do you think it might work with
dilute H2SO4? If that is the case, I might be able to make it one pot, as the reaction I have in mind is acid catalysed.
The reaction of terminal propynes with HgSO4 yeilds methyl ketones... not so much of a scheme really, just one step... I could draw out the detailed
mechanism with the intermediates if you'd like, but the overall reaction is simplicity itself.
R-CH2-C#C-H ------- HgSO4/ H2SO4 -------> R-CH2-CH(=O)-CH3
And of course R could be anything ; )
The interesting question then is the best synthesis of the terminal alkyne from existing and readily availiable, preferably OTC reagents, and i'm yet
to come up with that.... then i'll have another 2-propanone strategy to be proud of!
|
|
|
Flip
Hazard to Others
  
Posts: 116
Registered: 7-12-2005
Member Is Offline
Mood: No Mood
|
|
Synthesis of methyl ketones from terminal alkynes
Just to give you an example of what i'm talking about, here is a reaction that comes directly out of an Org Chem textbook...... (file is attached)
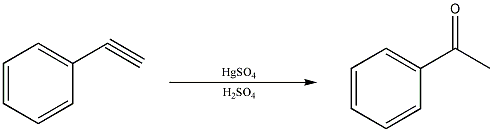
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Only hot concentrated H2SO4 reacts with mercury, since its oxidising properties are needed. The reaction gives off SO2, not hydrogen.
Though you might be able to use a relatively small amountof conc. H2SO4 to heat with the mercury, and use the resulting H2SO4/HgSO4 mix in your
reaction after dilution. There is no need to isolate HgSO4 in substance, which is difficult anyway.
If you have no HNO3 you can always distill your own.
Thanks for the reaction schemes, they are interesting.
Remind me of the preparation of acetaldehyde from acetylene (Chisso process), it uses a HgSO4 catalyst as well.
What are the necessary conditions for the conversion? Heat, reflux, etc...? How is the product isolated?
[Edited on 4-3-2006 by garage chemist]
|
|
|
Flip
Hazard to Others
  
Posts: 116
Registered: 7-12-2005
Member Is Offline
Mood: No Mood
|
|
Electrophilic addition of water to alkynes
Yeah, it is a great reaction. Sorry I can't be more helpful, as I've never run it myself and I haven't had too much time to research it - but i'd
like to give it a go once I finish with a few other projects. Here's the breakdown on the reaction:
 (Markovnikov regiochemistry) (Markovnikov regiochemistry)
And here's what looks to be a similar reaction I pulled up really quick off of orgsyn:
http://www.orgsyn.org/orgsyn/pdfs/CV5P0320.pdf
I can't figure why the nitrogen would be neccessary for the addition of water to alkynes... see that here that they start with cyclohexene... they had
to bring the solution to reflux to even get a reaction... but it was the best that I could come up w/ on short notice (sorry). I don't see any reason
why the nitrogen would be neccessary, but if it must be done it should be easy enough to get a small tank and a regulator. In any case ~50% yeild for
the scale they were working with would be excellent.
Now who's going to help me come up with a novel preparation of terminal propynes from non-suspect reagents?
[Edited on 4-3-2006 by Flip]
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
| Quote: | Originally posted by Flip
Now who's going to help me come up with a novel preparation of terminal propynes from non-suspect reagents?
|
What about your reaction you had earlier, use acetylene and NaNH2 in NH3 in a 1-1 stoichiometric ammount, then the reaction with alkyl halide to get
the terminal alkyne.
propyne is already available OTC, as MAPP gas, with a few other contaminants. It should be easy to get pure by making silver methylacetylide with the
gas cylinder, then treating the SMA with acid to get the pure propyne gas.
|
|
|
Dr.Muto
Harmless

Posts: 17
Registered: 2-3-2006
Member Is Offline
Mood: No Mood
|
|
Hg&H2SO4=HgSO4 via sublimation I think
|
|
|
Flip
Hazard to Others
  
Posts: 116
Registered: 7-12-2005
Member Is Offline
Mood: No Mood
|
|
That would be the most constructive use of MAPP gas that i've ever had. lol.
The thought had crossed my mind with acetylene. I didn't realize that MAPP was propyne. Wow, thanks. However, this sounds like it could be a bit
dangerous. MAPP gas makes me woozy. I guess I could try it outside. Acetylene and 1-(bromomethyl)-somethingorother could work nicely though. I was
just hoping to stay away from supply houses as much as possible.
[Edited on 4-3-2006 by Flip]
|
|
|
Flip
Hazard to Others
  
Posts: 116
Registered: 7-12-2005
Member Is Offline
Mood: No Mood
|
|
Solubility of HgSO4
Now here's something interesting...
My CRC Handbook of Chemistry and Physics, 82nd edition, lists under the solubility column "reac H2O". Chemfinder lists under solubility,
"decomposes".
I take this to mean that it will decompose to the element in H20. How will this affect the reaction... considering that what we have in mind here is
the addition of water to alkynes. It must be this H20 reactivity that drives the reaction, but as I understood it, the HgSO4 catalysed the
reaction, and was regenerated. Perhaps this is the function of the H2SO4, but does this mean that I won't be able to recover the HgSO4 post rxn? Now
I must admit i'm a bit confused... can anyone explain how this might affect the procedure?
[Edited on 4-3-2006 by Flip]
|
|
|
Flip
Hazard to Others
  
Posts: 116
Registered: 7-12-2005
Member Is Offline
Mood: No Mood
|
|
Well, I was looking into the HgCl2 thread and I remembered something that made me smack my forehead. HgI2 just made things too easy, I remember now
that HgSO4 is actually an intermediate in the HgCl2 synth published by Zygoat on Rhodium:
| Quote: |
[1] HgSO4
Measure 20g Hg and place in a 100ml conical flask. Do not use a round bottom flask, as all the mercury will not react. Add 60 ml concentrated H2SO4
(should be at least 94%) to the flask and fit a single hole stopper with a tube leading outside. This reaction produces lots of SO2 that will give you
chemically induced asthma if breathed. Now slowly heat the flask. Bubbles of SO2 will rise from the acid/mercury interface. Maintain a vigorous
bubbling of SO2 by adjusting the heat. A white crystalline deposit of HgSO4 will appear. The mercury should be completely reacted after about 30
minutes. Allow the reaction mixture to cool and pour off the acid. Pour the crystals/acid into 750 ml hot water and filter. Keep the liquid.
|
So I'm now one step closer to having this all put together.
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Flip
Now here's something interesting...
My CRC Handbook of Chemistry and Physics, 82nd edition, lists under the solubility column "reac H2O". Chemfinder lists under solubility,
"decomposes".
I take this to mean that it will decompose to the element in H20. |
No. It means that HgSO4 is not soluble in neutral/basic media and an insoluble yellow basic salt will precipitate. This salt can be redisolved by
acidifying the solution. This phenomenon is typical for Hg-salts.
Generally when a method calls for a HgSO4 solution, and HgSO4 is not available in powder form, people will just suspend an equimolar amount of HgO in
water, add H2SO4 dropwise under stirring until practically all is dissolved and filter off the remaining unsolubles (this is not strictly necessary
though).
If solid HgSO4 is needed, heating metallic Hg in H2SO4 until evolution of SO2 stops, cooling and finally decanting works fine, but take care to pipe
the SO2 through water and beware of the suckback (SO2 is *very* soluble in water).
|
|
|