DFliyerz
Hazard to Others
  
Posts: 241
Registered: 22-12-2014
Member Is Offline
Mood: No Mood
|
|
Phosphoric Acid from TSP
I'm giving myself a pretty dumb project which is synthesizing phosphoric acid from trisodium phosphate, and I've narrowed the process down to two main
equations:
2 Na3PO4 + 3 CaCl2 --> Ca3(PO4)2 + 6 NaCl
Ca3(PO4)2 + 3 H2SO4 --> 2 H3PO4 + 3 CaSO4
However, I've run into two problems with my process: the trisodium phosphate I have is 20-25% sodium sesquicarbonate, which will cause some calcium
carbonate to precipitate along with the tricalcium phosphate, so I need to separate the sodium sesquicarbonate from the trisodium phosphate
beforehand. Next, I don't have any clean sulfuric acid, but just have sodium bisulfate. That means that theoretically I'll have soluble sodium sulfate
in my phosphoric acid unless I can find a way to remove it beforehand. I know that this is a pretty dumb project, but I'd like to know if there are
any ways to fix these problems, even if it makes it extremely time/money inefficient.
|
|
|
j_sum1
Administrator
       
Posts: 6230
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
Well, if you really have time on your hands, I have a similar project on the go.
I have no H2SO4 except that which I make myself and so I have refrained from using that. I do have plenty of HCl that I can use.
I place a layer of Ca3(PO4)2 on the bottom of a plastic container with a sealable lid. Inside I place an open container of concentrated HCl. I put
the lid on and wait a few months.
After that time the HCl vapour has reacted with the calcium phosphate to form CaCl2 and phosphoric acid. The mixture is a sludge that oozes with the
acid. You can get some to decant off but you probably want a better method with higher yield. It would probably respond reasonably well to vacuum
filtration. The method I used was to dissolve the phosphoric acid in acetone, leaving the calcium chloride behind. The acetone was then distilled
off.
Unfortunately I picked up quite a bit of contamination in my first batch -- It was left sitting for a week with the acetone in an unsuitable
container. Still, it was enough for a proof of concept. I expect that my next batch will come out quite clean.
As a process, it is not real elegant, but it will fix both your problems.
|
|
|
DFliyerz
Hazard to Others
  
Posts: 241
Registered: 22-12-2014
Member Is Offline
Mood: No Mood
|
|
What about the sodium sesquicarbonate in the TSP?
|
|
|
j_sum1
Administrator
       
Posts: 6230
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
So, you end up with some calcium carbonate in with your calcium phosphate.
When exposed to the HCl, the carbonate will convert to CaCl2 giving off some carbon dioxide. The phosphate also converts to CaCl2 so there is no real
difference.
|
|
|
Texium
Administrator
       
Posts: 4520
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
How can you be so sure that the HCl will react in an appreciable way with the calcium phosphate though? It seems to me like that reaction would tend
to favor the other side and be very inefficient for phosphoric acid production. Now I'm curious about this, and when I get home I'm going to try
adding some phosphoric acid to a calcium chloride solution so see if a precipitate will form. If so, it would seem unlikely that the reverse reaction
would work for producing phosphoric acid.
|
|
|
DFliyerz
Hazard to Others
  
Posts: 241
Registered: 22-12-2014
Member Is Offline
Mood: No Mood
|
|
I'd kinda prefer not to wait a few months, are you certain there's no way to do it with sodium bisulfate or some other homemade sulfuric acid?
|
|
|
j_sum1
Administrator
       
Posts: 6230
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
Quote: Originally posted by DFliyerz  | | I'd kinda prefer not to wait a few months, are you certain there's no way to do it with sodium bisulfate or some other homemade sulfuric acid?
|
You could probably work out a way. I'm just letting you know what I did. It was mostly a way of doing something useful with some calcium phosphate
before I had any equipment or much else n the way of reagents. It worked. (I think it helped that temperatures were quite high over summer.)
@zts16
You tell me. I ended up with a thick acid with a slight yellow tinge. It was soluble in acetone and had a high boiling temperature. The powder that
accompanied it was not soluble in acetone but was soluble in water.
I'll admit, I did not attempt to obtain a precipitate from the acid and calcium chloride. But, as you say, that is the opposite of what I was
attempting to achieve.
|
|
|
Texium
Administrator
       
Posts: 4520
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by j_sum1  |
@zts16
You tell me. I ended up with a thick acid with a slight yellow tinge. It was soluble in acetone and had a high boiling temperature. The powder that
accompanied it was not soluble in acetone but was soluble in water.
I'll admit, I did not attempt to obtain a precipitate from the acid and calcium chloride. But, as you say, that is the opposite of what I was
attempting to achieve. |
Well, I stand corrected after testing it. I added about a mL of concentrated
phosphoric acid to a few mL of calcium chloride solution and it remained perfectly clear with absolutely no precipitation. I guess the greater
strength of HCl overrides the low solubility of the calcium phosphate in this scenario. It seems like a plausible method as long as the acid is
distilled afterwards.
|
|
|
j_sum1
Administrator
       
Posts: 6230
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
Plausible if you have a lot of time at your disposal.
Hey, I have a bucket with a lid and a spare spot on my bench. It isn't costing me anything.
|
|
|
macckone
International Hazard
    
Posts: 2159
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
You don't distill phosphoric acid. The acetone technique seems like a good separation method.
|
|
|
j_sum1
Administrator
       
Posts: 6230
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
If you are impatient you could always speed up the rate using a fluidised bed of your phosphate and pass HCl gas through.
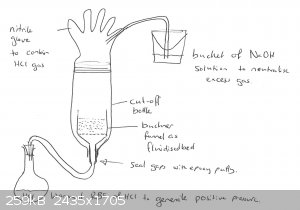
|
|
|
Texium
Administrator
       
Posts: 4520
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
My apologies,
I forgot about the polymerization/glass etching that comes with hot concentrated phosphoric acid.
|
|
|
chloric1
International Hazard
    
Posts: 1071
Registered: 8-10-2003
Location: GroupVII of the periodic table
Member Is Offline
Mood: Stoichiometrically Balanced
|
|
Why not just TSP and excess HCl
I’d like to make the point that sodium chloride is virtually insoluble in concentrated hydrochloric acid. So the sedimentation of NaCl will drive
the equilibrium to the right:
Na3PO4 + 3HCl >>>3NaCl + H3PO4.
I’m thinking the yield would be atrocious unless a later acetone separation was used. Personally, I’d prefer to separate disodium phosphate from
a TSP solution only partially neutralized to pH 8-9 by HCl since it has such low solubility in cold water. Also dosodium phosphate dodecahydrate is
so easy to melt and you could just add the remainder of the hydrochloric acid to that and just get a viscous slurry on heating. When excess HCl is
removed, add acetone
Fellow molecular manipulator
|
|
|
unionised
International Hazard
    
Posts: 5105
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
I'm sure I read somewhere that adding ethanol to sodium bisulphate gives a precipitate of sodium sulphate and a solution of sulphuric acid in
alcohol.
|
|
|
chloric1
International Hazard
    
Posts: 1071
Registered: 8-10-2003
Location: GroupVII of the periodic table
Member Is Offline
Mood: Stoichiometrically Balanced
|
|
Quote: Originally posted by unionised  |
I'm sure I read somewhere that adding ethanol to sodium bisulphate gives a precipitate of sodium sulphate and a solution of sulphuric acid in
alcohol. |
I’ve read the same thing numerous times. I feel similar about trisodium phosphate. Seems like TSP is effectively dibasic sodium phosphate and
sodium hydroxide. Sodium hydroxide is soluble in ethanol and especially methanol. Maybe you could remove the alkali from TSP leaving the dibasic
salt? Not sure.
Fellow molecular manipulator
|
|
|