ssdd
Hazard to Others
  
Posts: 211
Registered: 13-4-2007
Location: Central Canada
Member Is Offline
Mood: Hypergolic
|
|
Crazy Idea - What do you think?
I was having an interesting conversation the other day and I came up with a neat idea.
Most modern solar cells are made from materials that convert light at one peak color of light. If these panels were able to pick up light over a
larger range of colors more light could be converted to electricity thus having a higher efficiency rate. (I was searching for the frequency of light
that most cells peak out at but had some problems finding it, if anyone knows please inform me.)
Now I was thinking this: Our eyes absorb light on all visible frequencies. (Actually a combination of blue, green, and red.) Now If the chemicals from
our eyes (or other animals) could we perhaps use them as a type of solar cell which should have a greater efficiency level.
From what I can gather these chemicals would need to be regenerated over time and replaced. (This is far fetched but you could have bacteria generate
the chemicals much as we use bacteria to generate insulin.)
I have a copy of Gray's Anatomy sitting next to me and I'm reading more about the eye to see what I can gather up, but I was just wondering how crazy
you guys would think I am. 
-ssdd
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Eyes contain photosensitive chemicals, but as far as I know, they are NOT of interest in transferring the energy.
Do read up on plants, however. They're quite good at it -- they've been going strong for the last two billion years or so (dating back to seas of
cyanobacteria).
Tim
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
It's being handled by making the PV cells responsive to more wavelengths
http://www.google.com/search?hl=en&safe=off&q=multib...
Another approach is to use other power conversion methods instead of photovoltaics, such as thermal driven generators of one sort or another. More
efficient thermoelectric materials would really help with this.
And Tim is correct, the photochemistry of eyes is neither good at transforming light to useful power, nor is it easy to maintain outside of a living
animal.
If you look at the response curves, both the scoptic and photopic response curves of the human eye aren't hat wide, both are only about 100 nm wide at
the 50% point and roughly 150 nm at the 25% points. Modern solar cells have a wider response curve than that.
|
|
|
cool_arrow
Harmless

Posts: 15
Registered: 27-6-2005
Member Is Offline
Mood: No Mood
|
|
take a look at this link:
http://www.prismsolar.com/index.html
|
|
|
ssdd
Hazard to Others
  
Posts: 211
Registered: 13-4-2007
Location: Central Canada
Member Is Offline
Mood: Hypergolic
|
|
Yea after sitting and reading a bit more last night I see that as everyone pointed out this wouldn't work all to well. (Not to mention cost
effectiveness!)
Now the thing with plants is that they seem to not absorb green light. If I recall correctly green is the peak emission color of the sun. (Plants do
most of their light conversions on violet and red.)
Off topic but why would they not use green?
Thanks for the links they turned up some cool results and cool_arrow that site was pretty neat.
-ssdd
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
That's something that's always stumped me a little. There are dark plants, but I don't know that they perform any photosynthesis on the green that's
absorbed (i.e. it's just pigmentation).
Probably, they have never had an incentive to do so. CO2 is relatively scarce in the atmosphere. Wind blowing through a forest doesn't carry too
much CO2 (currently, 0.04%), and after a few miles, it contains even less (though I don't know how much less; CO2 absorbtion is probably a slow and
self-limiting thing with plants).
Now, if we blew coal burning exhaust into miles of greenhouses, maybe some plants would develop the heat resistance and efficiency to maximize growth.
The problem is that Darwin is slow.
Tim
|
|
|
Eclectic
National Hazard
   
Posts: 899
Registered: 14-11-2004
Member Is Offline
Mood: Obsessive
|
|
I read, or heard on one of the Discovery channels, that the green of chlorophyll may just be an evolutionary accident. Apparently there are, or have
been, other photosynthetic pigments that are red, purple, and other "odd" colors. Chlorophyll may just have an evolutionary edge regarding efficiency
or availability of required trace elements.
|
|
|
Twospoons
International Hazard
    
Posts: 1282
Registered: 26-7-2004
Location: Middle Earth
Member Is Offline
Mood: A trace of hope...
|
|
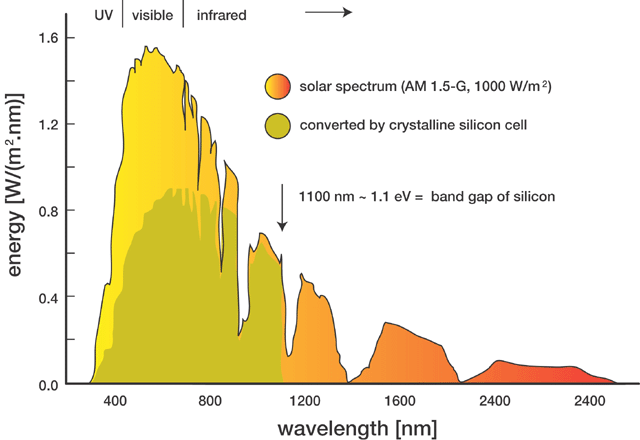
There's as much energy in the IR as there is in the visible.
Green doesn't make up that much of the total energy output.
[Edited on 25-6-2007 by Twospoons]
Helicopter: "helico" -> spiral, "pter" -> with wings
|
|
|
indigofuzzy
Hazard to Others
  
Posts: 145
Registered: 1-10-2006
Location: DarkCity, Bay of Rainbows, Moon
Member Is Offline
Mood: Distilled
|
|
Since silicon's bandgap is around 1100 nm, and there are transparent semiconductors with larger bandgaps, couldn't a double layered solar cell produce
more efficiency? Put the transparent semiconductor on top, and silicon on the bottom.
<pre>
-----------------------------
| SiC P-doped layer |
----------------------------- SiC -- frequently used in Blue LEDs,
| SiC N-doped layer | bandgap should be aroun 470nm.
----------------------------- <--- conductive SnO<sub>2</sub> or similar
----------------------------- (this layer avoids a P-N junction being formed
| Si P-doped layer | between dissimilar semiconductors.)
-----------------------------
| Si N-doped layer |
-----------------------------
| Metal substrate |
-----------------------------
</pre>
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by indigofuzzy
Since silicon's bandgap is around 1100 nm, and there are transparent semiconductors with larger bandgaps, couldn't a double layered solar cell produce
more efficiency? Put the transparent semiconductor on top, and silicon on the bottom.
... |
Done there, been that:
single junction multigap
http://emat-solar.lbl.gov/research/Multiband.html
http://www.trnmag.com/Stories/2004/042104/Material_grabs_mor...
decent overview of multijunction and multigap research
http://gcep.stanford.edu/pdfs/solar_workshop_10_04/SolarWalu...
|
|
|
YT2095
International Hazard
    
Posts: 1091
Registered: 31-5-2003
Location: Just left of Europe and down a bit.
Member Is Offline
Mood: within Nominal Parameters
|
|
I could be wrong, but I know with Laser power testing it`s common to use LEDs and a current meter, Phosphide and arsenide green and red LEDs
respectively for the laser beam color, so green LED is used for a green laser etc...
now this May or Maynot have anything to do with anything, but when you think of how White LEDs work, perhaps it may be possible to incorporate another
Layer that absorbs and re-emits at a frequency the panel is more responsive to.
turning some of this wasted UV into visible light.
just a thought 
\"In a world full of wonders mankind has managed to invent boredom\" - Death
Twinkies don\'t have a shelf life. They have a half-life! -Caine (a friend of mine)
|
|
|
cool_arrow
Harmless

Posts: 15
Registered: 27-6-2005
Member Is Offline
Mood: No Mood
|
|
I've often wondered why so much emphasis is put on the efficiency of PV's when when the cost has to be just as big an issue. What if the the
efficiency stayed at their current levels but costs were dramatically reduced? If a company announced that they had figured out how to manufacture
these things at a fraction of what the currently cost I think that would be big news. Perhaps there are some limits to how cheap the materials to
mfr. can be sold?
|
|
|
YT2095
International Hazard
    
Posts: 1091
Registered: 31-5-2003
Location: Just left of Europe and down a bit.
Member Is Offline
Mood: within Nominal Parameters
|
|
well I currently produce (no pun intended) 12v at 5 amps with the combined power of my PV panels, so I`de welcome ANYTHING that could lower the price
for the same product!
\"In a world full of wonders mankind has managed to invent boredom\" - Death
Twinkies don\'t have a shelf life. They have a half-life! -Caine (a friend of mine)
|
|
|
Twospoons
International Hazard
    
Posts: 1282
Registered: 26-7-2004
Location: Middle Earth
Member Is Offline
Mood: A trace of hope...
|
|
The emphasis on efficiency is relevant to cells intended for space use, where reductions in launch weight are really important.
For terrestrial use, where there is plenty of room (like on a roof), then cost really is the critical factor. Or, more correctly, $ per watt over
lifetime. No good having a cheap cell that only lasts a week!
The other factor is we really need a manufacturing process that consumes much less energy than the cells can produce over their life, otherwise we are
wasting our time.
Helicopter: "helico" -> spiral, "pter" -> with wings
|
|
|