halogen
Hazard to Others
  
Posts: 372
Registered: 18-4-2004
Member Is Offline
Mood: No Mood
|
|
formation of cyclopropanones from alkene?
I've been thinking about the production of cyclopropanones from alkenes lately, but I can't find too much information on it. The use of carbon
monoxide as the carbene would seem to be largely ineffective for several obvious reasons. The two ideas that I am pondering are:
1. Use the standard dichlorocarbene (in situ from chloroform and NaOH) And then somehow convert -C(Cl2)- to -CO-. Possibly NaOH but that seems
unlikely.
2. 2-chloro-dioxolane -O-CH2-CH2-O-CHCl and NaOH instead of chloroform to form a spiro compound with the alkene. Then, later, the ethylene glycol can
be removed by hydrolysis to leave the cyclopropanone unit.
There are a few shortcomings however, some of them quite obvious. How can I get a >C=O from >CCl2 ??? And would the 2chlorodioxolane even react
with NaOH (or other strong base) to form the necessary carbene? Furthermore, many of the shorter alkenes are gasseous! That provides a whole other
problem.
One of my aims is cyclopropanone. If I start with acrylic acid which is a liquid, I might end up with the carboxylic acid (w/ obvious ketone). I could
try to eliminate the CO2 with heat, but that might end up breaking my nice 3-ring or it could expand the ring to some sort of cyclobutanone or
something... Which would be interesting in it's own right. Also, acrylic acid is an acid. It would clearly neutralise my strong base so that no
carbene would even form! I wonder if it is possible to perform the same cycloaddition with an acrylate salt?
edit
And I forgot another problem: how to create 2-chlorodioxolane! (Provided what I was thinking was even possible, this would be an even grander
challenge) Oxolane is used as an industrial solvent, so there must be some way of making it. Chemically, it is a cyclic ether; composed of
formaldehyde (methylene diol) and ethylene glycol. However, the 2-chloro bit would be interesting to see about, as I imagine it would interfere with
the etherisation, and even then, formyl chloride has never been synthesised. Furthermore, I would think 2chlorodioxolane would readily react with
water, which would be necessary to dissolve the NaOH. Unless hydrolysis is significantly slow...
This is turning out to be quite a dilemma.
[Edited on 18-7-2007 by halogen]
|
|
|
halogen
Hazard to Others
  
Posts: 372
Registered: 18-4-2004
Member Is Offline
Mood: No Mood
|
|
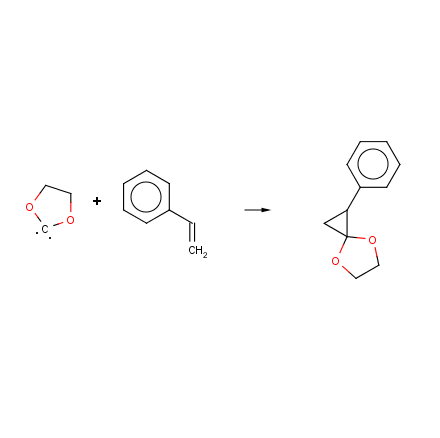
I have used something called MarvinSketch for a quick illustration of the reaction between 2 chloro dioxolane and NaOH modeled after the standard
chloroform+NaOH trick. (in situ, with phase transfer catalyst of course).
[Edit]
The first pic was screwed up. This second one is the reaction between an alkene (here, styrene) and my fancy carbenoid. Notice the spiro product?
This can then be hydrolysed to ethylene glycol and the desired product (Phenylcyclopropanone (?) ) Other substitutions would be equally possible. Of
course, how likely is the carbenoid to catalyse the polymerisation to polystyrene?
[Edited on 23-7-2007 by halogen]
F. de Lalande and M. Prud'homme showed that a mixture of boric oxide and sodium chloride is decomposed in a stream of dry air or oxygen at a red heat
with the evolution of chlorine.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
There is also the resonance problem that could push pull the the opening of the cyclopropanone and hexring closure to a cyclohexenone --> favourise
a less stressed bicyclo compound Beta-tetrahydronaphtone like.
CH CH2
// \ / \
CH CH CH2
| | |
CH C C
\\ / \\ / \\
CH CH O
[Edited on 7-8-2007 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
It is theoretically possible to synthesize a cyclopropanone directly by combining an alkene and CO under sufficient heat and pressure, probably with
the aid of a solid catalyst on the surface of which the reaction takes place, with the C of the CO adding across the C=C double bond. Because two
volumes of gas or vapor would be combining to make one, such a reaction would be favored by high pressure.
It is well-known that the CH2: diradical, and substituted methylenes such as :CCl2, when liberated in decomposition reactions (e.g. by decomposition
of CHCl3 in the presence of an ionic alkoxide, or by thermal or photo-decomposition of di-iodides or of diazomethane and similar diazo compounds)
react with alkenes to form cyclopropane derivatives. However, CO is a diradical, :CO, in only one of its two resonance forms, the other being the
polar (-)C[triplebond]O(+) form (a combination of a carbanion and a tertiary oxonium cation), which may affect matters.
I cannot find any reference to such a reaction with CO in my textbooks, or on the internet, so this reaction possibility would be a good research
topic for someone.
|
|
|
|