m_turner_02
Harmless

Posts: 1
Registered: 19-10-2007
Member Is Offline
Mood: No Mood
|
|
predicting degree of unsaturation
i have a problem due in my organic class and i have a question on how to predict the degree of unsaturation of the molecule: CH3N2 the teacher
said it is an anomoly, how?
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Google diazomethane. It is usually given as CH2N2. It readily yields carbene and N2 gas. It is yellow, explosive and methylates DNA (or most other
nucleophiles).
Then google degree of saturation. This is frequently done via titration with Br2.
Cheers,
O3
[Edited on 19-10-2007 by Ozone]
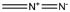
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
that extra proton had me looking too... was that a typo?
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Diazomethane is not CH3N2! There is no molecule with CH3N2 empirical formula, it can only fit to some ions and radicals. Your teacher either made a
typo or is not talking about a molecule, but a transient species, radical or ion.
If he made a typo and meant CH2N2 instead, there are a couple of compounds that fit that empirical formula: diazomethane and cyanamide.
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
I'm affraid we're mssing his deadline but what is being asked? The 'degree of unsaturation' is not a concept I've heard, at least not that way. Is
he asking how many double and or triple bonds? I'd have to assume so but I don't think it's clear. Second, let's assume it's not a typo that the
empirical formula is CH3N2. He did say it's an anomaly and cyanamide and diazomehtane would not usually be considered anomalous.. uniqe maybe but
within bounds, huh? So where does that leave us? A transition species of some kind as nicodem stated earlier. A radical or ion.
If H3CN2 there would be a formal charge on the central N. It would not be a stable compound but might it occur as a transition state?
|
|
|
jam640
Harmless

Posts: 29
Registered: 3-10-2007
Location: Rainbow Island
Member Is Offline
Mood: Fat
|
|
The degree of unsaturation is a very simplified way of calculating the amount of multiple bonds present in a molecule.
First you need to determine how many hydrogens your molecule should have if fully saturated, i.e CnH2n+2 for alkanes.
If halogens are present you add 1 hydrogen for each. Oxygens are ignoed and the number of nitrogens are subtracted from the total amount of hydrogens.
THen it is just a simple matter of subtract your calculated amount of hydrogens from the number of hydrogens that is present in the fully saturated
compound. Then each 2 hydrogens correlates to 1 degree of unsaturation.
So for CH2N2:
CnH2n+2 = 4
4 - 2nitrogen = 2
4 -2 = 2 = 1 degree of unsaturation
This is classic undergrad stuff, and any introductory textbook should cover it. However it is not very useful.
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
| Quote: | Originally posted by Ozone
Then google degree of saturation. This is frequently done via titration with Br2. Titrating vs. Br2 (endpoint is persistence of Br2 color) gives the
mole eq. of sp2 or sp carbon present via vic. addition to form the di or tetra bromo derivatives (per bond segment), respectively.
Yes, CH2N2 is a hybridized dipole.
[Edited on 19-10-2007 by Ozone] |
I did not want to do all of his/her homework.
Also of interest, "hyperconjugation"!
Cheers,
O3
[Edited on 21-10-2007 by Ozone]
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|