azo
Hazard to Others
  
Posts: 163
Registered: 12-2-2008
Member Is Offline
Mood: No Mood
|
|
aryloxozolines
I no there is old threads about this topic but a long time ago and with some unanswered questions that someone might be able to put some light on.
The cyclization of the isomers 1r.2s and 1s/2r of arylaminoalcohols with potasium cyanate yeilds trans aryloxozolines which i can understand because
the amine group is facing in the same direction as the hydroxy group allowing cyclization to take place.
But with the 1s.2s and 1r 2r isomers they have the hydroxy group facing opersite the amino group so i couldn't see how cyclization could take place
without some form of inversion.
I am in the understanding that the 1s.2s and 1r,2r isomers go to the oxozolidinones ? Is this correct or does somehow the 1s.2s and 1r.2r produce cis
aryloxozolines.
REGARDS AZO
[Edited on 13-5-2011 by azo]
|
|
|
Nicodem
|
Thread Moved
13-5-2011 at 09:05 |
azo
Hazard to Others
  
Posts: 163
Registered: 12-2-2008
Member Is Offline
Mood: No Mood
|
|
? whats going on here. I posted a question about organic chemistry and it ends up in beginings i didnt think it was that simple ,the last post a wrote
on this got removed completly.
I think it least deserves a responce
By the way i am not a drug cook and have got no intension of being one .I don't want to end up in jail at my age of 50 that would be dumb.
I just like the chemistry SO DON'T CALL ME ONE OR TREAT ME AS ONE
regards azo
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
Quote: Originally posted by azo  | | ? whats going on here. I posted a question about organic chemistry and it ends up in beginings |
Quote: Originally posted by Nicodem  | Please open threads without references, and/or with vague or unanswerable questions only in the Beginnings section.
|
|
|
|
azo
Hazard to Others
  
Posts: 163
Registered: 12-2-2008
Member Is Offline
Mood: No Mood
|
|
To clear things up the starting aminoalcohol is phenylpropanolamine but i haven't been able to find a mechanism for the cyclization of
phenylpropanolamine with cyanate salts to aryloxazolines.
There is a lot of info in regards to the cyclization with cyanogen bromide,but the mechanism is different because with cyanogen bromide norephedrine
would yield the cis isomer but using a cyanate salt it would yield mostly the trans isomer.
Can someone please explain the difference of these mechanisms and tell me if the 1r 2r and the 1s 2s isomers of phenylpropanolamine produces
aryloxazolinones or the corrosponding cis aryloxazoline.
hope this helps my question
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
I know nothing of the mechanism of this rxn, but the alcohol probably epimerizes or the reaction goes through a carbocation intermediate (if it is
true that only trans is produced by the rxn).
"Titanium tetrachloride…You sly temptress." --Walter Bishop
|
|
|
GreenD
National Hazard
   
Posts: 623
Registered: 30-3-2011
Member Is Offline
Mood: Not really high anymore
|
|
Can you give a picture of what ones you are talking about?
|
|
|
azo
Hazard to Others
  
Posts: 163
Registered: 12-2-2008
Member Is Offline
Mood: No Mood
|
|
Thanks for the reply smuv and green d .
Here is a diagram of the 4 isomers of 4 mar,but i am unable to give a mechanism for the cyclization of phenylpropanolamine with cyanate salts , If i
had a mechanism it could put some light on whether norpsheudoephedrine affords cis 4 mar or aryloxazolinones.There is very little references to this
rxn so i thought i would have to put it to the many brilliant people from this site to give there thoughts on it.
I hope now that it is in the beginers section that it would still get answered by the members that would normaly post in the organic section.
many thanks azo
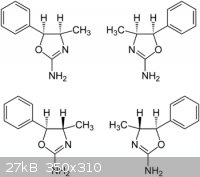
|
|
|