| Pages:
1
2
3 |
Psychopatic
Harmless

Posts: 8
Registered: 9-8-2004
Location: Sweden
Member Is Offline
Mood: Insane
|
|
Most corrosive agent?
Hello everyone! I'm new here!
Now, my first question is, how to manufacture the most corrosive agent as possible. I have concentrated Nitric Acid (68%) & concentrated
Hydrochloric Acid (35%), in my larder. And this gives the well-known Aqua Regalis, in the properties 1:3. But is it possible to make something even
more corrosive than this? I also have concentrated Sulphuric Acid (96%).
Hydroflourine Acid, is said to be extremly corrosive, but I do not have access to that.
Any tip?
|
|
|
svm
Harmless

Posts: 31
Registered: 21-7-2004
Member Is Offline
Mood: No Mood
|
|
here's a tip: don't use HF. it's terribly, terribly toxic.
it is very, very corrosive too, however.
|
|
|
Hang-Man
Hazard to Self
 
Posts: 70
Registered: 16-1-2004
Location: Canada
Member Is Offline
Mood: Wasted
|
|
Bah. Someone delete this before I have an aneurism.
1. It’s hydofluoric acid, not ‘Hydroflourine Acid’
2. It’s aqua regia not ‘Aqua Regalis’
3. How the hell do you measure how ‘corrosive’ something is.
4. It doesn’t matter because this will be deleted before you read my response.
Here's a tip, look into super acids/bases.
|
|
|
JustMe
Hazard to Others
  
Posts: 111
Registered: 7-8-2003
Member Is Offline
Mood: No Mood
|
|
What Hang-Man said!
However, impure HF could probably be made with your Sulfuric acid and the mineral Fluorite (which is easily available). I wouldn't try it...
insidiously toxic, and (hopefully you already know) it corrodes glass.
On the other hand if you could get potassium or sodium dichromate and combine it with salt and sulfuric acid then you get chromyl chloride.
Argh, somebody stop me! I'm incorrigible and incorriging him.
|
|
|
kyanite
Hazard to Self
 
Posts: 86
Registered: 30-6-2004
Location: is not important!
Member Is Offline
Mood: lactamic
|
|
http://www.powerlabs.org/chemlabs/hydrofluo.htm
at 70% it dissolved some of a test tube, and brought the acid to a boil
[Edited on 9-8-2004 by kyanite]
|
|
|
Psychopatic
Harmless

Posts: 8
Registered: 9-8-2004
Location: Sweden
Member Is Offline
Mood: Insane
|
|
Well, sorry about my english, it's not so good, especially not in technical terms. But I hope you all know what I mean!
Well, I think I skip the Hydofluoric Acid, I know it's very toxic.
But the dichromate-thing sounds interesting. I think I have ammonium-dichromate, mayby it will work as well?
And I know it's difficult to measure how corrosive substances are. But I mean, if you put the agent on, for example iron, how fast it will
destroy the material.
|
|
|
raistlin
Hazard to Others
  
Posts: 200
Registered: 5-7-2002
Location: Ohio
Member Is Offline
Mood: No Mood
|
|
For the sake of playing devils advocate...
He's from Sweden, which my be the reason for saying "hydroflourine" rather than "hydroflouric".
Aqua Regalis is correct, as it comes directly from Latin, mean- 'Royal Water' or 'Water of Kings'. However, the far more common
way of hearing it said, is indeed, aqua regia.
Or then again the guy could quite possibly be a total moron and me defending him is pretty pointless...
\"To ignite, or not to ignite, that is the question.\"
|
|
|
Quantum
Hazard to Others
  
Posts: 300
Registered: 2-12-2003
Location: Nowhereville
Member Is Offline
Mood: Interested
|
|
Is it just me
or should you learn some basic chemistry before messing with stuff that has a huge potential of hurting you?
What if, what is isn\'t true?
|
|
|
Hang-Man
Hazard to Self
 
Posts: 70
Registered: 16-1-2004
Location: Canada
Member Is Offline
Mood: Wasted
|
|
| Quote: |
And I know it's difficult to measure how corrosive substances are. But I mean, if you put the agent on, for example iron, how fast it will
destroy the material. |
Consider, dilute nitric acid will attack copper but concentrated Hydrochloric acid will not. This isn't because dilute nitric is 'more
corrosive' but because it is an oxidizer as well as an acid. HCl will also attack some things nitric acid will not, (pure Al?)
So if you want to measure how corrosive it is you must also state what it is attacking. Hot H2SO4 will eat organic matter faster than anything else
(molten lye would probably be comparable) but wouldn't be as effective on metal as some hydrogen halides.
As for steel, Aqua Regia would most likely be best, or some other acid/oxidizer mix. I have used HCl/H2O2 on hard metals with success. But Aqua Regia
won’t eat things like silver where as nitric acid alone will, so specify the target.
I knew Aqua Regia was known as royal water but didn't know regalis was correct, nor did I see he was foreign. All I saw was shitty English and
posts 1. Although I still see Vultures circling on this thread.
[Edited on 9-8-2004 by Hang-Man]
|
|
|
Saerynide
National Hazard
   
Posts: 954
Registered: 17-11-2003
Location: The Void
Member Is Offline
Mood: Ionic
|
|
With your "gimme the worst thing you got" type of post, I really hope you aren't intending to do anything harmful to others with this
acid you want to acquire 
"Microsoft reserves the right at all times to monitor communications on the Service and disclose any information Microsoft deems necessary to...
satisfy any applicable law, regulation or legal process"
|
|
|
Psychopatic
Harmless

Posts: 8
Registered: 9-8-2004
Location: Sweden
Member Is Offline
Mood: Insane
|
|
No, no! I would definitely not harm anyone. I just want a "cool substance" that will "eat upp things" 
And yes, it is true that I don't have advanced chemistry-knowledges. But, I'm intressted of it, reading a lot of books and pages on the
net about it, mainly about organic chemistry. And I also work part-time at a pharmacy, so have access to many chemicals 
|
|
|
Reverend Necroticus Rex
Hazard to Others
  
Posts: 196
Registered: 15-6-2004
Location: Right behind you.......
Member Is Offline
Mood: Poisonous
|
|
There is always "magic acid" fluoroantimonic acid, a 50% solution of SbF5 in HF, vicious stuff, pretty much the strongest superacid in
existence I think.
The sun is shining on a brand new day
Blackened corpses burn where they were slain
Self-flagellation prompts him to confess, Bless me father, for I made this mess.
|
|
|
Organikum
resurrected
    
Posts: 2337
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
corrosive sublimate
thats it. 
Its also more poisonous than Zyankali. So say some books. Others say otherwise, its not healthy - ok? 
And its not water-incompatible. As most supa-acids.....
|
|
|
guy
National Hazard
   
Posts: 982
Registered: 14-4-2004
Location: California, USA
Member Is Offline
Mood: Catalytic!
|
|
Also, HSO3F and SbF5 mixture gives a super acid.
And is perchloric acid stronger than sulfuric acid?
[Edited on 9-8-2004 by guy]
|
|
|
Democritus of Abdera
Harmless

Posts: 47
Registered: 5-8-2004
Location: The Prefecture of Abdera in the region of Xanthi.
Member Is Offline
Mood: Stone Dead
|
|
http://www.sciencemadness.org/talk/viewthread.php?tid=1791
Caro's Acid picked up the nickname of Pirahna Bath after one witty chemist noticed it eating a candle someone had placed in a beaker of
it.
It also has the benefit of being made from common and inexpensive reagents.
Do not meddle in the affairs of wizards, for you are crunchy and taste good with ketchup.\"--- Marcel Marceau
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
As regards gases, fluorine gas is undoubtedly the most corrosive, especially when there is water vapor also present; or as a vapor, platinum
hexafluoride (rather unstable though) which can even abstract electrons from O2, N2 and Xe to form salts.
As regards stable liquids, I would think a mixture of HNO3 and HF. I believe that this can dissolve Pt, unlike aqua regia (HNO3 + HCl) which dissolves
Au but not Pt.
John W.
|
|
|
DDTea
National Hazard
   
Posts: 940
Registered: 25-2-2003
Location: Freedomland
Member Is Offline
Mood: Degenerate
|
|
Just adding my bit of warning to what's already been said... Describing HF as simply "toxic" is not adequate. HF is extremely
damaging to the bones and causes painful, slow-healing wounds. Do not go anywhere near it if you don't have Calcium Gluconate gel.
Wounds from HF may lead to death or limb amputation!!!
"In the end the proud scientist or philosopher who cannot be bothered to make his thought accessible has no choice but to retire to the heights in
which dwell the Great Misunderstood and the Great Ignored, there to rail in Olympic superiority at the folly of mankind." - Reginald Kapp.
|
|
|
BromicAcid
International Hazard
    
Posts: 3241
Registered: 13-7-2003
Location: Wisconsin
Member Is Online
Mood: Rock n' Roll
|
|
HClO4 (>75%)/H3PO4 mixture at its boiling point will eat glass and metals and detonate if it comes into contact with organic material.
|
|
|
Reverend Necroticus Rex
Hazard to Others
  
Posts: 196
Registered: 15-6-2004
Location: Right behind you.......
Member Is Offline
Mood: Poisonous
|
|
Bromic, won't concentrated perchloric acid
detonate in contact with organics wether in the prescence of H3PO4 or not?
How about tellurium, isn't H2TeO4 supposed to be a stronger acid per % than H2SO4? I imagine peroxymonotelluric acid would be yet more powerful
still.
Can H2O2 exist in solution with HF? I'm not sure, but seing as H2O2 isn't exactly going to oxidise HF due to the lack of F oxyacids, than
would a strong solution of HF in H2O2 be possible, and one hell of an oxidiser if it is.
The sun is shining on a brand new day
Blackened corpses burn where they were slain
Self-flagellation prompts him to confess, Bless me father, for I made this mess.
|
|
|
budullewraagh
Hazard to Others
  
Posts: 168
Registered: 1-8-2004
Location: new york
Member Is Offline
Mood: Aliphatic
|
|
| Quote: | | There is always "magic acid" fluoroantimonic acid, a 50% solution of SbF5 in HF, vicious stuff, pretty much the strongest superacid in
existence I think. |
a 50% solution of SbF5 in H5 is 10^18 times as acidic as 100% H2SO4.
peroxymonohydrosulfuric acid is also extremely acidic.
if you are asking for the "most powerful acid," are you referring to the acid that has the lowest pK1? but then again we have to factor in
the possibility of a pK2, pK3, pK4, etc.
|
|
|
JustMe
Hazard to Others
  
Posts: 111
Registered: 7-8-2003
Member Is Offline
Mood: No Mood
|
|
Well, I'm glad that somebody went into more detail about HF. Anyway, I don't think ammonium dichromate can be substituted in making Chromyl
Chloride (in an ALL GLASS apparatus, like a retort). Though it makes a neat fake volcano, of course. 
Another very corrosive material is Chromium Trioxide (need we remind us that Hexavalent Chromium is nothing to play around with casually). This can be
made from sodium dichromate and concentrated sulfuric acid... look it up.
But it all depends on what you want to corrode. And it begs the question that if it meant to corrode "anything," what are you gonna keep it
in? (And for how long?) 
Please read, read and read. Start simple. Many years ago I also had a similar situation... access to chemicals through a part-time job at a pharmacy.
Ah, them was the days!
|
|
|
BromicAcid
International Hazard
    
Posts: 3241
Registered: 13-7-2003
Location: Wisconsin
Member Is Online
Mood: Rock n' Roll
|
|
| Quote: |
Bromic, won't concentrated perchloric acid
detonate in contact with organics wether in the prescence of H3PO4 or not?
|
Very true, especially concentrated solutions when boiling. I just added the H3PO4 in the mix because concentrated phosphoric acid, when hot, will
attack glass, something perchloric will not do on its own. This is not a special solution, just something mixture in my head that someone would throw
out with only a little chemistry.
Superacids are indeed very acidic by pKa standards but how do they react when exposed to virgin metal? Is the ferocity of their attack in parallel to
their intense pKa? However they do dissolve long chain hydrocarbons so they must have something going for them.
Molten CsOH would be quite a formidable corrosive, readily attacking glass and other materials.
Bromine pentafluoride supposedly reacts with every element known except the lower noble gasses and nitrogen directly. Explodes on contact with water
and organics, just another 'corrosive' compound.
As stated though, it all depends on what criteria fit your definition of corrosive.
|
|
|
Blind Angel
National Hazard
   
Posts: 845
Registered: 24-11-2002
Location: Québec
Member Is Offline
Mood: Meh!
|
|
Maybe this scheme could help understand pKa and acidity scale
(old one i know  ) )
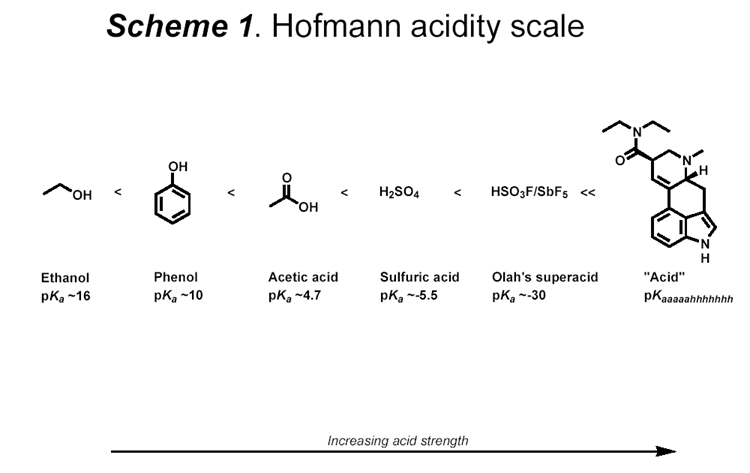
/}/_//|//) /-\\/|//¬/=/_
My PGP Key Fingerprint: D4EA A609 55E4 7ADD 8529 359D D6E2 33F6 4C76 78ED |
|
|
budullewraagh
Hazard to Others
  
Posts: 168
Registered: 1-8-2004
Location: new york
Member Is Offline
Mood: Aliphatic
|
|
| Quote: | | Superacids are indeed very acidic by pKa standards but how do they react when exposed to virgin metal? Is the ferocity of their attack in parallel to
their intense pKa? However they do dissolve long chain hydrocarbons so they must have something going for them. |
in a superacid, the HF has (just about) completely dissociated leaving a ton of H+ wondering what the hell happened to their respective electrons. as
a result, these H+ decide to protonate things, namely hydrocarbons because they can easily take, oh, say dodecane and form 12 methane molecules. it
makes the H+ happier and the hydrocarbons dont even know what hits them. as for metals, a similar effect happens. the hydrogen is not so happy
without any influence on any electrons so it jumps on metals (even if they aren't so active) because hey, there is a better chance of them
getting control of an electron than from SbF6- seeing as the F- are all crazy about the Sb+5.
|
|
|
Psychopatic
Harmless

Posts: 8
Registered: 9-8-2004
Location: Sweden
Member Is Offline
Mood: Insane
|
|
Quantum:
I'm knowing what i'm doing, and I have done experiments with much more dangerous chemicals than HF.
Reverend Necroticus Rex:
I've heard about this Fluoroantimonic acid, I think it is ment in the Guinnes Records of the World.
JustMe:
I have a very small pot with Chromium Trioxide! This should be examinated... There is no warning signs on it.
Working at the pharmacy is fun! But the boss is not so happy about my orders  .
But it is my dad who is boss there, so... .
But it is my dad who is boss there, so...
Some chemicals I have got from there (the most hazardous):
Metallic Sodium
Bromine
White Phosphorus (this was not something to play with!)
Potassium Cyanide
Mercury Chloride
Arsenic
Thallium
I also worked at the hospitals physics-department, as a trainee. Some radioactive isotopes "mystically disappeared", from there 
|
|
|
| Pages:
1
2
3 |