| Pages:
1
2
3 |
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
The idea of shielding the plates from the direct heat might help, that never occurred to me. I will try that out as I still have some silica powder
left. As for the sandpaper I'm not sure that is necessary... Etching might be an idea I could try via bifluoride or HF, but I'm not convinced that a
rough surface is needed.
BTW, forgot to mention that the slides were cleaned in conc. ammonia, rinsed in dH2O and air dried prior to coating.
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
Mailinmypocket. You're plates might be too thick. Ensure your gypsum is dehydrated before using it. An hour at 350*F-400*F should be okay more would
probably be better. I've also read that using methanolic solutions of the slurry can allow it to be used/stored indefinitely rather then a one time 5
minute race before preparing more slurry.
I heard an old trick for making uniform thickness TLC plates is to take a glass stirring rod wrap a few layers of tape(uniformly) on both ends. Think
a bar-bell. Then roll it over the microscope slide/glass-plate. I believe common thicknesses for stationary phases for TLC plates are 350-450uM? To me
that sounds like one layer of tape above the height of the plate.
If you think about it from a surface point of view. If your plate is too thick the adhesion between the weekly bound silica particulates will be
strong enough to disrupt and adhesion between the slide and the surface.
I also know that rinsing plates in pirhana solution is used in preparing 'lab on a chip' type applications when bonding PDMS. It works by oxidizing
the surface. So an acetone rinse to remove oils, then DI to remove acetone, then hydrogen peroxide instead of ammonia sounds good to me. Or go in for
the kill and prepare some dilute pirhana solution. Ammonia will de-protonate the silanols, which I'm not sure if that's advantageous for this. I'm not
sure how long the deprotonation would hold up after rinsing in D.I. water anyways. It may take up available spots for hydrogen bonding thus weakening
the surface cohesion.
Maybe I'm over thinking it. Either way I'll be joining you on this adventure this weekend, maybe by working together we can some up with something
that works really well.
[Edited on 15-8-2013 by smaerd]
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
So I kind of blew it with the applicator idea. One height of duct tape is not enough. The glass stir rod happily adhered to the slurry. Should have
seen that one coming. Maybe a few layers of tape instead of one would be a lot better. Hard to say but 1 ply was way too thin.
The best plate I made today so far was the one without using any kind of spreader(1 out of 5). I did realize some of the issues however.
Lessons learned:
1) It appears imperative that the silica gel and microscope slides are oven dried thoroughly before application. A detail I likely missed on my last
adventure.
2) dehydrating gypsum at 300*F(~150*C) for an hour works fine for dehydration.
3) Use a mortar and pestle and a razor blade to mix the plaster of paris into the silica gel. This is the only way I've found to get a uniform solid
mixture.
4) Working fast is annoying. Hardly have time to stir the slurry before it sets. I think using a mixture of ethanol/methanol/isopropanol and water
would give us a lot more time to lay the plates.
5) The amount of water in the slurry seems critical. This will be what defines the density of the colloid. Too much and it's too thick, too little and
its useless. I shouldn't have deviated so much from the recommended 2x by weight, woops.
6) Air bubbles. Make sure you tap your plate(s) several times before letting them dry.
I'll try another experiment tomorrow using an alcoholic slurry.
[Edited on 18-8-2013 by smaerd]
this little publication has some information about alcohol slurries and making a simple applicator. good information base. Contains information about
fluorophores and sorbents etc. -
http://85.238.144.18/lifescience/literature/061009_Making_TLC_Plates_from_Bulk_TLC_Silica_Gels.pdf
apparently polyvinyl alcohol can be used to make stronger plates rather then gypsum. That'd be interesting.
[Edited on 18-8-2013 by smaerd]
Ah-hah! Commercial plates use what is called a 'hard-layer' rather then a soft-layer(gypsum binder). The hard layer is a polymeric binder IE polyvinyl
alcohol or similar... These plates will probably always be fragile. To be fair though I have had 'nice' layers. You just can't really write on them.
Reference: Handbook of Thin-Layer Chromatography By Sherma/Fried page 162 (see google books).
I'll give this a shot, polyvinyl alcohol is cheap especially considering it only requires 1-2% of the weight of the stationary phase. Probably has a
more reasonable set time as well (heat activation not time).
[Edited on 18-8-2013 by smaerd]
[Edited on 18-8-2013 by smaerd]
|
|
|
IrC
International Hazard
    
Posts: 2710
Registered: 7-3-2005
Location: Eureka
Member Is Offline
Mood: Discovering
|
|
This patent contains useful information related to depositing thin films on glass.
METHOD FOR DEPOSITING A THIN LAYER AND PRODUCT THUS OBTAINED
"Science is the belief in the ignorance of the experts" Richard Feynman
|
|
|
sonogashira
National Hazard
   
Posts: 555
Registered: 10-9-2006
Member Is Offline
Mood: No Mood
|
|
I've been experimenting with talc as a stationary phase with very good results. There are some good guides for the preparation of TLC plates using
talc. I have never seen it mentioned as a stationary phase for chromatography in any book, but the results from a few trials that I have done using
radial chromatography and small column chromatography have been very impressive, as are the reported separations in the papers attached (one of which
is too big, and is therefore not attached, but is here: http://pubs.acs.org/doi/abs/10.1021/ed044p294). In fact it is the best paper, so I have uploaded it here: http://www.sendspace.com/file/8rha7a
The paper concerning porphyrin chromatography discusses the preparation of the plates (from a slurry with methanol) and the effectiveness of the
separation. It's all very straightforward, and the results (in general) are sufficient for solvent selection purposes, at the very least. As mentioned
in the papers, more favourable results than silica gel may be obtained for some systems, and it would likely serve as a good alternative to confirm
the results from a silica system, in cases where alumina is not suitable (if you bother to do so, anyway!).
Attachment: Talc TLC plates.pdf (644kB)
This file has been downloaded 804 times
Attachment: Talc as a TLC adsorbent.pdf (108kB)
This file has been downloaded 1317 times
Attachment: Porphyrin TLC using talc.pdf (646kB)
This file has been downloaded 615 times
[Edited on 29-8-2013 by sonogashira]
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
That is very interesting! Thanks for sharing!
Up until now (since smaerd's experiment results) I have been messing around with polyvinyl alcohol(PVA) and etched plates hoping for better adhesion.
Had to put it on hold due to some pretty nasty burns from saturated ammonium bifluoride.... Just kidding! 
Haven't had the time to experiment lately... Only had time to etch the plates and prepare the PVA solution. This method with methyl cellulose and talc
intrigues me, I have methyl cellulose but no talc yet, have you any experience using unscented baby powder? Or have you only used pure talc powder?
|
|
|
sonogashira
National Hazard
   
Posts: 555
Registered: 10-9-2006
Member Is Offline
Mood: No Mood
|
|
I just used what I found in the bathroom. Imperial leather original talc. I mixed it with methanol (2 g talc to 3 ml methanol, stoppered and shaken
for 1 minute in a 4 ml volumetric flask), poured onto a glass plate, and left it to dry overnight. The layer adheres to the glass when held vertically
(and up side down), but I have been using horizontal development so far, just to test the resolution. To get a consistent thin (250 um) layer, the
dipping method described in the porphyrin paper seems suitable, but I will get some pure talc before I try that. Hopefully I can remove commercially
prepared plates from my shopping list entirely. The method that I described is amply suitable for solvent selection experiments using radial
chromatography, though, and the results translate onto commercially prepared silica plates. It depends on one's intended use. I do more qualitative
tests than quantitative. I am going to try to impregnate a piece of chromatography paper with talc, using a methanol suspension. I also bought some
cab-o-sil fumed silica for this purpose, but I have a feeling that talc will better-adhere. Another paper on preparing talc TLC plates (using
ethanol): http://link.springer.com/article/10.1007/BF02330381
I should mention that, in contrast to silica plates, talc does not need to be activated prior to using. It is debatable whether silica plates need
activation, I suppose, but that's a different matter. A picture of one of the plates used either for radial chromatography or drop chromatography (10
cm diameter plate). This one was left nearest the window in my shed, so there are a few more minor ripples than on the others, which were almost
entirely flat:

[Edited on 31-8-2013 by sonogashira]
|
|
|
sonogashira
National Hazard
   
Posts: 555
Registered: 10-9-2006
Member Is Offline
Mood: No Mood
|
|
I could only find two samples to test. I was a little heavy-handed, but the resuls are good, regardless. There are two samples in the picture: the
yellow one to the left is just-about visible, and the orange one was developed with two different solvents (IPA and ethyl acetate respectively, from
left to right). I've scraped some of the talc off so that the uniform thickness may be seen, as well as the ease with which one may make clean edges
due to the softness of the talc, especially compared with silica.

As mentioned above, this is common bathroom talc (scented!). I used 2 g of talc, 3 ml of methanol (giving 4 ml of talc-slurry), air-dried on a flat
plate for only 30 minutes before spotting.
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
I actually did find that the thinner the stationary phase on gypsum and silica plates the tougher it was so my hypothesis seems right.
I just got some poly vinyl alcohol in this week so I'll try and make some plates with it if I have time in the next coming days and report back.
|
|
|
sonogashira
National Hazard
   
Posts: 555
Registered: 10-9-2006
Member Is Offline
Mood: No Mood
|
|
I tried ascending chromatography on an aluminium-supported talc plate, and the part that was in the solvent (ethyl acetate) fell off. I don't know
whether it's the talc or the solvent that was to blame, but a 'concentration zone' of diatomaceous earth would be advisable if using ascending
chromatography with talc. The resolution was excellent, however, and the portion falling-off didn't affect the separation in any way. Perhaps the
methyl cellulose additive will help. Or just do radial chromatography by dropping solvent into the centre with a capillary tube: http://www.youtube.com/watch?v=9poQ8AVlbgc
Visualizing the spots by spraying a solution of iodine in DCM onto the plate worked as well as for normal silica-plates, but the talc is too soft to
write upon with pencil. Hopefully this is of use to someone. I'm going to try mixing cab-o-sil fumed silica with the talc to see how it affects the
retention.
[Edited on 31-8-2013 by sonogashira]
|
|
|
sonogashira
National Hazard
   
Posts: 555
Registered: 10-9-2006
Member Is Offline
Mood: No Mood
|
|
I take it back. My thin layer was far too thick. By using a thicker suspension than before, and dipping an aluminium plate into it repeatedly, a very
very smooth thin layer can be produced just with talc that will not fall off with any solvent that I have tested. The appearance and uniformity is
every bit as good as a commercial silica plate. When I have an impure sample to test I will take a picture of it developed side by side with a
commercial silica plate.
[Edited on 2-9-2013 by sonogashira]
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
Nice sonogashira. In the one .pdf I posted they said for making small quantities of plates you can take two plates back to back and dip them into
solution. Remove them and separate and viola two plates ready made. Probably by next-weekend I'll be preparing some plates and working on my other
project.
|
|
|
seba
Harmless

Posts: 16
Registered: 27-11-2011
Location: SI
Member Is Offline
Mood: No Mood
|
|
What about using PVA (wood glue) as the binder?
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
Completely forgot to update my experimental efforts here.
I have tried a few variations with polyvinyl alcohol(PVOH). I have gotten close but nothing quite there.
I experimented with sillica to 5-10% by mass hydrolyzed polyvinyl alcohol. The basic procedure so far has been as follows:
*Abrade microscope slides with sandpaper(also tested without abrasion without much effect). Clean microscope slides with acetone and oven dry.
*Add PVOH to a small volume of distilled water. Warm water to about 70-80*C until complete dissolution.
*Oven dry silica gel for column chromatography. Let cool, and then weigh appropriate proportion.
*Made slurry with silica gel by adding the PVOH solution and mixing. Think this is where things go wrong...
*Apply slurry too microscope slides so there is no drips over-hanging (Important)
*Let the slides sit, then oven dry gently(have experimented with this a bit).
Now the problem I have been having is a bit unexpected. I appear to get a great TLC plate. Well until I touch it. It seems as though the PVOH forms a
'bubble'. Meaning The outer edge is dry and bonded exactly how I would like. Even a crusty polymeric film layer exists on the face of the plate. When
touched with a pencil or similar however, this 'bubble' shatters to reveal dry mobile silica gel underneath.

It's as if the PVOH concentrates to the outer edges of the TLC plate and does not fully mix with the sillica slurry no matter how much it is mixed.
I'm not sure why this may be the case. I'm wondering if it has to do with hydrogen bonding being more favorable to the silica surface from the water,
rather then PVOH to the the silica. I think trying isopropanol as a solvent may then prove to be more fruitful? Another alternative would be to do
something similar to PCR's thermal phase. Warm the slurry to 80-90*C so the thermal motion over-comes the strength of the hydrogen bonding, then allow
the solution to cool. I will try in the future and update if there is success. Acquiring some input would be fantastic.
I think PVOH would be a great layer if it does work. PVA(polyvinyl acetate) would be great as well, but it may pollute the plate with acetates?
Probably be preferred to use lab-grade PVA and not the yellow gunky stuff. Might be cross-linkable in an easy way to get a nice firm PVOH/PVA layer.
[Edited on 26-5-2014 by smaerd]
[Edited on 26-5-2014 by smaerd]
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
From the book - Thin Layer Chromatography in Chiral Separations and Analysis by Teresa Kowalska, I found another recipe for TLC plates used in chiral
analysis. I'd imagine this could be substituted for regular silica gel rather then the chiral one.
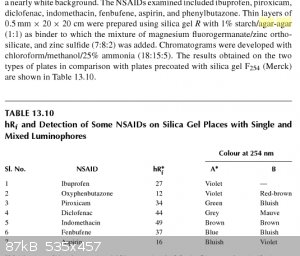
|
|
|
Sulaiman
International Hazard
    
Posts: 3678
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
don't know if it helps but for plate photography, spreading an even film of emulsion is necessary and here is one applicator that could be modified
http://thelightfarm.com/Map/DryPlate/PlatePrep/DryPlatePart4...
|
|
|
| Pages:
1
2
3 |