| Pages:
1
..
6
7
8
9
10
..
80 |
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Regarding "Myth Busters": This is entertainment, not scientific research.
It CAN be fun to watch sometimes, but the more you know, the more you see them mess up.
They allways jump from testing hypothesis to "LET'S BLOW SOMETHING UP!" to a hasty, dumbed down conclusion, instead of collecting a meaningful amount of data and analyzing it properly!
[Edited on 7-4-2014 by Bert]
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
underground
National Hazard
   
Posts: 702
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Yea that is true, they just do whatever they must to do to have as much viewing as possible, that is all, they do not care about nothing more.
[Edited on 7-4-2014 by underground]
|
|
|
Dany
Hazard to Others
  
Posts: 482
Registered: 3-8-2013
Member Is Offline
Mood: No Mood
|
|
The experiment is serious. For those who don't know the man with "Mythbusters" team, his name is Dr. Van ROMERO:

He is a known detonation physicist, he done a lot of research on detonation of high explosives. He was the vice president of new mexico Institute of
Mining and Technology.
Dany.
|
|
|
underground
National Hazard
   
Posts: 702
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
So why do they use ANFO and not somethink like TNT?
|
|
|
Dany
Hazard to Others
  
Posts: 482
Registered: 3-8-2013
Member Is Offline
Mood: No Mood
|
|
For all the hasty members (moderator included) who directly replied to Dornier post by saying that the
experiment is not serious and not scientific...Well, ladies i'm affraid to tell you that the experiment is very scientific and serious...As i posted
above, Dr. Van ROMERO who conducted the experiment is a known detonation physicist and he know exactly what is he doing. First, lets
go back to the video posted by Dornier . One might ask, why they used a coaxial metal tube for conducting the experiment? as one can
see at the beginning of the video, Dr. Van ROMERO is holding a coaxial metal tube, then they put the carbon in the inner tube. The
reason is described in two US patent .
US3823044 A: Increasing the detonation pressure of ammonium nitrate/fuel oil compositions
&
US3667911 A: Method of treating solids with high dynamic pressure
Quoted from US3823044 A:
"In the shock synthesis of diamond from non-diamond carbon by the proces described in US. Pat. 3,667,911, issued June 6, 1972, to A. S. Balchan
and G. R. Cowan, a spanning or transverse shock wave is introduced into non-diamond carbon positioned in a metal container cylinder, by the
progressive collision of the container cylinder and a surrounding coaxial metal driver cylinder, which is propelled by a detonation, the shock wave
moving axially through the non-diamond carbon at a velocity equal to the collision velocity, or the detonation velocity of the explosive surrounding
the driver cylinder. The detonation velocity preferably i in the range of about from 3000 to 6000 meters per second, most preferably about from 4000
to 5000 meters per second. At the same time, to achieve optimum yields, the detonation pressure of the explosive needs to be sufficiently high to
accelerate the driver cylinder over the short distance between colliding cylinders at a sufliciently high velocity to produce the cylinder-spanning
shock wave"
in the same patent (US3823044 A) they added:
"Although the desirability of employing ANFO mixtures in a process of this kind is great when factors such as safety, economy, and convenience are
considered, the ANFO mixtures heretofore known which detonated in the required velocity range did not develop a sutficiently high detonation pressure
to afford optimum yields in the process. Heretofore, higher detonation pressure could be achieved only at the expense of increasing the detonation
velocity to a value outside the required range."
As normal ANFO cannot generate the required pressure for converting carbon to diamond they made a special ANFO mixture for increasing the pressure of
detonation. This ANFO mixture is suitable for diamond synthesis via the coaxial metal tube described above. Also from US3823044 A:
"The ANFO detonating composition of this invention, which comprises AN in the size blends specified above as well as the previously specified
amount of densifying agent, has a bulk density of at least about 1.00 gram per cubic centimeter, and an ideal detonation pressure, calculated for a
composition containing 10% densifier, ranging from about 35 kilobars at a detonation velocity of 4000 m./sec. to about 60 kilobars at a detonation
velocity of 5000 m./sec., these pressures and velocities being suitable for the diamond synthesis process described in US. Pat. 3,667,911, the
disclosure of which patent is incorporated herein by reference"
After all this explanation, members should be more scientific when they criticize experiment done by an expert like Dr.Van ROMERO.
Learn to read and search before replying randomly.
Dany.
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
 Yay! Documentation!! Referances AND pertinent quotes from same!!! Patent
information!!!! Yay! Documentation!! Referances AND pertinent quotes from same!!! Patent
information!!!! 
This is the best documented thing posted here in a while-
Dany, you are not like the rest... You make me happy.
 Not like some of the others, who make tiny plastic baby Jesus cry, and kill many
kittens. Not like some of the others, who make tiny plastic baby Jesus cry, and kill many
kittens.
I still think Jaime and Adam are fucking entertainers not scientists, and the show is "infotainment" at best- But their guest was legit, it would
appear.
[Edited on 7-4-2014 by Bert]
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
Do you think the tetrasodium salt of tetranitroaminoethane actually have a detonation velocity of 10.9km/s ? This is so much higher than ONC. around
30% by weight of this explosive produces Na2O, and it will react with CO2 to produce sodium carbonate, so there are only few gas moles left, how is
10.9km/s possible?
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
Has anyone tried synthesis of hydroxylazide? (N=N=N-O-H) Hydroxylazide must be a strong acid and will give the anion azidooxide -1.
It may be synthesized from reduction of Nitrylazide(N=N=N-NO2) or Nitrosylazide (N=N=N-N=O)
Or maybe It can be synthesized from H-O-Cl + NaN3 > H-O-N=N=N + NaCl?
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
HOCl splits like HO(-) and Cl(+); NaN3 splits like Na(+) and N3(-)... so how would you join Na(+) and Cl(+) or HO(-) and N3(-)? You should get tiny
amount of Cl-N3 and NaOH and finally NaOCl + HN3.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Dornier 335A
Hazard to Others
  
Posts: 231
Registered: 10-5-2013
Location: Northern Europe
Member Is Offline
Mood: No Mood
|
|
Yes, hypochlorous acid and sodium azide solutions react to form extremely explosive chlorine azide gas.
Woelen has done it: http://www.sciencemadness.org/talk/viewthread.php?tid=10581
|
|
|
FeRe
Harmless

Posts: 8
Registered: 14-4-2014
Member Is Offline
Mood: No Mood
|
|
For example Tatp is a static sensitive explosives. For its is able to go off through static discharge, a human is needed ?(can it just go off through
a human who has enough static electricity) I mean can it go off itself through static discharge when it is in a tube.
|
|
|
Dany
Hazard to Others
  
Posts: 482
Registered: 3-8-2013
Member Is Offline
Mood: No Mood
|
|
FeRe, you are asking this question because you synthesized TATP and you are afraid that the tube will go off by itself or between your hand...wright?
a small advice boy, stay away from peroxide and sensitive explosive, your parent need you and you need to keep your hand safe...
Dany.
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
Quote: Originally posted by PHILOU Zrealone  |
HOCl splits like HO(-) and Cl(+); NaN3 splits like Na(+) and N3(-)... so how would you join Na(+) and Cl(+) or HO(-) and N3(-)? You should get tiny
amount of Cl-N3 and NaOH and finally NaOCl + HN3. |
Thanks, I realized it too after I commented.
What do you think will happen upon NO2+ nitration on hydroxylamine? Will it yeild N-hydroxydinitramide? I final product seems stable, I am worried
about one intermediate compound - N-hydroxynitramide.
|
|
|
FeRe
Harmless

Posts: 8
Registered: 14-4-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Dany  | FeRe, you are asking this question because you synthesized TATP and you are afraid that the tube will go off by itself or between your hand...wright?
a small advice boy, stay away from peroxide and sensitive explosive, your parent need you and you need to keep your hand safe...
Dany. |
No I didn't I just wanted to explain myself with this example. You can replace tatp with lead azide or other static sensitive primaries. I know
peroxides are dangerous. Someone can help me in this question ?
[Edited on 14-4-2014 by FeRe]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by DubaiAmateurRocketry  | Thanks, I did not find its sensitivity until the referrence.
What about the Hyponitrite 2- anion? Again dihydroxylammonium hyponitrite seems interesting with perfect OB (NH3OH)2(N2O2). The hyponitrite anion
seems more stable than the nitroxylate.
Also what do you think about nitrosyl hyponitrite anion? the hydroxylammonium salt will be a isomer of ADN - N4H4O4, however this compound might be
denser due to the hydrogen bonds, and maybe more sensitive.
http://pubs.acs.org/doi/abs/10.1021/jp204967h?journalCode=jp...

[Edited on 30-3-2014 by DubaiAmateurRocketry] |
The making of hydrazinium or hydroxylamonium exotic oxonitrogen salts will be ruled by the oxydoredox potential of the anion vs the
cation...disproportionation reaction may result with destruction of both cation and anion.
Hydroxylamonium nitrite or hydrazinium nitrite (precursor of HN3) are good examples, just as ammonium nitrite which exists but is unstable and turns
into N2 and H2O.
NH4NO2 --> N2 + 2H2O
So the existance of an alkaline salt (Na/Li/K) is not per se meaning that an NH4, NH3OH or N2H5 salt could be done.
Not strictly related to nitroxylate or to hyponitrite... on the organic side there exist cupferron made by reaction of aromatic hydroxylamine with NH3
and nitrite ester...
Ar-NHOH + R-O-N=O + NH3 --> Ar-N(ONH4)-N=O + R-OH
This group of compounds has not been very much studied as energetic group.
Edited:
Just imagine that you have 1,3,5-trichloro-2,4,6-trinitrobenzene... the chlorine atoms are activated for substitution:
-reaction with water provides trinitrophloroglucidol
-reaction with NaN3 provides trinitrotriazidobenzene
-reaction with NH3 provides trinitrotriaminobenzene
-reaction with H2N-NH2 provides trinitrotrihydrazinobenzene
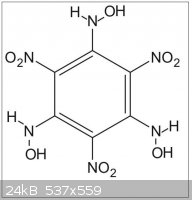
So maybe that reaction with hydroxylamine will provide a way to trinitrotrihydroxylaminobenzene (C6(NO2)3(NHOH)3)
--> Has someone info on that compound?
(see THATNB.jpg file joined)
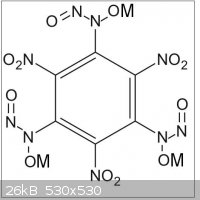
The later could then be reacted with ammonia and a nitrite ester to provide 1,3,5-trinitro-2,4,6-(N,N',N"-trinitroso-trihydroxylamino)-benzene
triammonium salt (C6(-NO2)3(-N(ONH4)-N=O)3 or C6H12N12O12).
Maybe this triammonium salt could precipitate valuable primaries with heavy metals, or with alkalies (Li, Na, K, ...).(see TNHATNB.JPG file joined
where M = heavy metal like Ag, Hg, ...)
Maybe the triammonium salt could exchange ammonium for hydrazinium (C6(-NO2)3(-N(ON2H5)-N=O)3 or C6H15N15O12) or for hydroxylamonium
(C6(-NO2)3(-N(ONH3OH)-N=O)3 or or C6H12N12O15)
(see TNHATNB.JPG file joined where M = NH3OH or NH3-NH2)....
[Edited on 16-4-2014 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by BobD1001  | I purchased a quantity of Formaldehyde from Elemental about 6 months ago. I received it in an opaque HDPE container, and it has since been stored
along other similar liquids within a storage cabinet in my semi-temp controlled garage. The minimum temperature this cabinet will see in the winter is
approximately 55* F. Upon taking inventory today, I noticed a thick layer of white residue on the bottom of the bottle. At first I thought the bottle
was losing its integrity and becoming porous, but I quickly realized that it was in fact a white solid at the bottom of the container. A quick search
reveals that this is most likely Paraformaldehyde forming due to the colder temperatures. My question is, upon warmer temperatures will this
redissolve to reform the formaldehyde solution, or will it require more elevated temperatures. Here is a link to a picture of the bottle and its
bottom layer. Link
|
No paraformaldehyde won't redissolve by temperature increase.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by DubaiAmateurRocketry  | Can this be nitrated?
http://en.wikipedia.org/wiki/Dihydroxyacetone
And, can someone help me find synthesis of dinitroethane? or trinitroglycerin? Just interested to see the impact sensitivity and other properties of
these liquid EM since the ONO2 group give much more impact sensitivity than NO2 group. I have searched on spinger and WOL, but not much related
contents are shown. Thanks. |
Dihydroxyaceton is not nitratable directly it is a strong reductor because in equilibrium with 2,3-dihydroxy-propanal...it has the ability to
polymerize into dark polyphenolic stuffs (see its use as artificial suntanner).
Just as aceton (propanone) is unstable towards HNO3, so would DHA.
It could be nitrated by a derivated way via war gas lacrymator dichloroaceton and reaction with silver nitrate saturated solution...
Cl-CH2-CO-CH2-Cl + 2 AgNO3 --> O2N-O-CH2-CO-CH2-O-NO2 + 2 AgCl (s)
The molecule might suffer hydrolysis behavior (and unstability-explosive runaway) because of the cetonic group and enol-ceton equilibrium. This effect
is seen in nitrosuggars (nitrate esters of suggars).
Dinitroethane can be 1,1 (geminal) or 1,2 (viccinal)... synthesis is pretty easy using the conventional synthesis pathways of nitroalcanes. I suppose
you want the 1,2-DNE.
R-Br or R-I + AgNO2 (or LiNO2/NaNO2/KNO2 in DMF).
Trinitroglycerin is a confusing name for nitroglycerin; I suppose you meant 1,2,3-trinitropropane.
There is indeed very little info on those nitroalcanes of the family H-(CHNO2)n-H.
Edited:
Stil they are of much interest because surprisingly at a certain point of n, the linear polynitroalcanes (with one NO2 per carbon unit (cf
nitromethane, 1,2-dinitroethane, 1,2,3-trinitropropane) surpasses in density equivalent lenght polymeric H-(CHONO2)n-H (with one ONO2 per carbon unit
(cf methyl nitrate, EGDN, NG, ETN, XPN, MHN) by much; and polymeric H-(CHNHNO2)n-H (with one nitramino per carbon unit (cf methylnitramine, EDNA,
propantrinitramine)) by a little.
So in theory it can lead to the highest density CHNO explosives this not counting with the fact each CH-NO2 unit:
-can react with formaldehyde to make (-C(CH2OH)(NO2)-)n which can be nitrated to make perfect OB polymer related to nitro-isobutyl-trinitrate ester
and 2,3-dinitro-2,3-dimethylol-1,4-butanediol tetranitrate ester (O2NOCH2-(-C(CH2ON2)(-NO2)-)n-CH2ONO2)
-can react with nitrous acid to introduce a nitroso group in the place of the hydrogen increasing by much the density.
One should not pernitrosate the compound in a way to get perfect OB and some H bondings...1 nitroso per 3 unit would be optimum.
-(CHNO2-CHNO2-CHNO2-)n + HNO2 --> (-C(NO)NO2-CHNO2-CHNO2-)n + H2O
Pernitrosated compound would be denser but would be overoxygenated and would require some extra dense fuel to burn.
[Edited on 16-4-2014 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by DubaiAmateurRocketry  | 10900 m/s? Is this even real? Tetranitroaminoethane's tetrasodium salt have a calculated detonation velocity of 10.9km/s at its crystal density! and
it is reported to have a VoD of 9570 at only 1.84g/cm3 I am trying to find for information if this is true. The tetraammonium, hydrazine,
hydroxylammonium salt of this also seems interesting.
I frist saw this russian paper.
Download link(press the pdf) = http://yadda.icm.edu.pl/yadda/element/bwmeta1.element.baztec...
I then searched for the english name and this paper poped up. This is a review, the tetrasodium salt of tetranitroaminoethane is on the last page.
quoted from a post slightly below:
Do you think the tetrasodium salt of tetranitroaminoethane actually have a detonation velocity of 10.9km/s ? This is so much higher than ONC. around
30% by weight of this explosive produces Na2O, and it will react with CO2 to produce sodium carbonate, so there are only few gas moles left, how is
10.9km/s possible?
|
The name is confusing tetranitroaminoethane should more be like ethynyltetranitramide, tetranitraminoethane or 1,1,2,2-tetra-nitramino-ethane
(2(O2N-NH-)2CH-CH(-NH-NO2)2).
It is kind of a dimer of methylenedinitramide (CH2(-NH-NO2)2) and as such it will display higher detonic performances data (d, VOD, brisance).
True that 9570 m/s at a density of 1,84 g/ccm seems a little too high, but maybe does the value come from calculations what might account for
overestimation.
Tetrasodium salt and other ammonium, hydazinium or hydroxylamonium salts will be energetic, no doubt...
But for the tetrasodium salt it is hard to tell the VOD because it is not a CHNO explosive...the presence of the Na will increase the density by a lot
but the sodium will be a dead weight in the end of the explosion process (as you said Na will take some oxygen and maybe catch some CO2 under cooling
and equilibration- this requires time and detonation is out of equilibrium reaction)...
However during the detonation process itself at the temperature of explosion sodium will behave like an ionised gas (a plasma) and will for sure
contribute to the shockwave (just like water what also displays explosive behaviour over 2000°C by splitting back to its elements H2 and 1/2 O2).
Sodium is linked to nitrogen and so during detonation maybe some metalic Na gas is produced (this happens with NaN3) so the heat of burning of Na
contributes to the temperature of explosion but less so than the original hydrogen atom.
(CH(NNaNO2)2)2 --> 2 CO2 + 2 Na2O +H2O + 4 N2 + 1/2 O2
So in the balance you have density increase vs heat of explosion loss.
As a side note in the second document at the end...the reaction is a nuclear reaction because the russians or the chineses reacted the
tetranitramino-ethane with calcium hydroxyde (Ca(OH)2) and they got the tetrasodium salt... so they turned calcium into sodium.   
Even if there is a translation error, chemistry remains chemistry.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
TheAlchemistPirate
Hazard to Others
  
Posts: 151
Registered: 25-3-2014
Location: The point of no return
Member Is Offline
Mood: Enigmatic
|
|
Hey guys I have lately been having troubles with distilling nitric acid, and was wondering if an oil bath is what I need. Basically, I have tried
synthesizing it by heating H2SO4 and KNO3 at 100 degrees C on my hot plate, but every time I do it the acid starts condensing on the sides of the
boiling flask(round-bottomed). I also noticed that when I moved the flask to one of the corners it started pressurizing, and almost shattered the
stopper that popped out because of it.
My theory is that Im heating it too much in order to make up for the lack of ambient heat around the flask. Anyways, I wanted to know how I can fix
this problem. If I were to place a stainless steel bucket filled with mineral oil on the hot plate would that work? Or will I have to spend another
200$ on a heating mantel? I haven't seen anywhere on google or youtube where anyone has done this.
"Is this even science anymore?!"
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
Quote: Originally posted by PHILOU Zrealone  | Quote: Originally posted by DubaiAmateurRocketry  | Can this be nitrated?
http://en.wikipedia.org/wiki/Dihydroxyacetone
And, can someone help me find synthesis of dinitroethane? or trinitroglycerin? Just interested to see the impact sensitivity and other properties of
these liquid EM since the ONO2 group give much more impact sensitivity than NO2 group. I have searched on spinger and WOL, but not much related
contents are shown. Thanks. |
Dihydroxyaceton is not nitratable directly it is a strong reductor because in equilibrium with 2,3-dihydroxy-propanal...it has the ability to
polymerize into dark polyphenolic stuffs (see its use as artificial suntanner).
Just as aceton (propanone) is unstable towards HNO3, so would DHA.
It could be nitrated by a derivated way via war gas lacrymator dichloroaceton and reaction with silver nitrate saturated solution...
Cl-CH2-CO-CH2-Cl + 2 AgNO3 --> O2N-O-CH2-CO-CH2-O-NO2 + 2 AgCl (s)
The molecule might suffer hydrolysis behavior (and unstability-explosive runaway) because of the cetonic group and enol-ceton equilibrium. This effect
is seen in nitrosuggars (nitrate esters of suggars).
Dinitroethane can be 1,1 (geminal) or 1,2 (viccinal)... synthesis is pretty easy using the conventional synthesis pathways of nitroalcanes. I suppose
you want the 1,2-DNE.
R-Br or R-I + AgNO2 (or LiNO2/NaNO2/KNO2 in DMF).
Trinitroglycerin is a confusing name for nitroglycerin; I suppose you meant 1,2,3-trinitropropane.
There is indeed very little info on those nitroalcanes of the family H-(CHNO2)n-H.
Edited:
Stil they are of much interest because surprisingly at a certain point of n, the linear polynitroalcanes (with one NO2 per carbon unit (cf
nitromethane, 1,2-dinitroethane, 1,2,3-trinitropropane) surpasses in density equivalent lenght polymeric H-(CHONO2)n-H (with one ONO2 per carbon unit
(cf methyl nitrate, EGDN, NG, ETN, XPN, MHN) by much; and polymeric H-(CHNHNO2)n-H (with one nitramino per carbon unit (cf methylnitramine, EDNA,
propantrinitramine)) by a little.
So in theory it can lead to the highest density CHNO explosives this not counting with the fact each CH-NO2 unit:
-can react with formaldehyde to make (-C(CH2OH)(NO2)-)n which can be nitrated to make perfect OB polymer related to nitro-isobutyl-trinitrate ester
and 2,3-dinitro-2,3-dimethylol-1,4-butanediol tetranitrate ester (O2NOCH2-(-C(CH2ON2)(-NO2)-)n-CH2ONO2)
-can react with nitrous acid to introduce a nitroso group in the place of the hydrogen increasing by much the density.
One should not pernitrosate the compound in a way to get perfect OB and some H bondings...1 nitroso per 3 unit would be optimum.
-(CHNO2-CHNO2-CHNO2-)n + HNO2 --> (-C(NO)NO2-CHNO2-CHNO2-)n + H2O
Pernitrosated compound would be denser but would be overoxygenated and would require some extra dense fuel to burn.
[Edited on 16-4-2014 by PHILOU Zrealone] |
Thank you PHILOU Zrealone for anwsering my question in this detail. Interesting, a polymer with positive oxygen balance would be extremely
interesting.
http://www.sciencemadness.org/talk/viewthread.php?tid=27095&...
In this post last reply, I uploaded a file on easily nitrating HTPB. Also for dinitroethane, is the carbon too acidic for use in propellant additives?
Also, what do you think about the nitronium cation nitration on HONH2? Will it yeild HON(NO2)2 ?
|
|
|
Davin
Harmless

Posts: 36
Registered: 5-12-2012
Member Is Offline
Mood: No Mood
|
|
Generally, the best reagent for the addition of an oxygen atom to a nitrogenous system is hypofluorous acid, HOF, prepared by the bubbling of a 10%
F2 in N2 mixture through H2O/MeCN at -10 oC. Reaction with sodium azide did not seem to work, but I only
tried a few times.
Would be amazing to undergo a cycloaddition with a nitrile giving a tetrazole-1-oxide, but alas it does not seem to exist.
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
Quote: Originally posted by Davin  |
Generally, the best reagent for the addition of an oxygen atom to a nitrogenous system is hypofluorous acid, HOF, prepared by the bubbling of a 10%
F2 in N2 mixture through H2O/MeCN at -10 oC. Reaction with sodium azide did not seem to work, but I only
tried a few times.
Would be amazing to undergo a cycloaddition with a nitrile giving a tetrazole-1-oxide, but alas it does not seem to exist. |
Ahh I almost forgot about the HOF-CH3CN system for adding oxides. This seems
Tetrazole-1-oxides and its derivatives actually do exist.
http://www.sciencedirect.com/science/bookseries/00652725/106
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
while trying to make high conc HNO3 using nitrate salts , the result is RFNA , this is because of the NOx mixtures.
to convert RFNA to WFNA we need to make vacuum distillation.
I was thinking to add few drops of distilled water on RFNA to increase its concentration using this equation :
3 NO2 + H2O → 2 HNO3 + NO
i.e, make a reaction between NO2 in RFNA and H20 to get some more HNO3. would that work ?
Ref : http://en.wikipedia.org/wiki/Nitric_acid
[Edited on 20-4-2014 by ecos]
|
|
|
thesmug
Hazard to Others
  
Posts: 370
Registered: 17-1-2014
Location: Chicago, Il (USA)
Member Is Offline
Mood: No Mood
|
|
I think it should work, but you're going to have to be very careful. You would need just enough water to increase the concentration as much as you
can, but not enough that you start diluting it.
|
|
|
NeonPulse
Hazard to Others
  
Posts: 417
Registered: 29-6-2013
Location: The other end of the internet.
Member Is Offline
Mood: Isolated from Reality! For Real this time....
|
|
Quote: Originally posted by TheAlchemistPirate  | Hey guys I have lately been having troubles with distilling nitric acid, and was wondering if an oil bath is what I need. Basically, I have tried
synthesizing it by heating H2SO4 and KNO3 at 100 degrees C on my hot plate, but every time I do it the acid starts condensing on the sides of the
boiling flask(round-bottomed). I also noticed that when I moved the flask to one of the corners it started pressurizing, and almost shattered the
stopper that popped out because of it.
My theory is that Im heating it too much in order to make up for the lack of ambient heat around the flask. Anyways, I wanted to know how I can fix
this problem. If I were to place a stainless steel bucket filled with mineral oil on the hot plate would that work? Or will I have to spend another
200$ on a heating mantel? I haven't seen anywhere on google or youtube where anyone has done this.[/
rquote]
Try insulating the boiling flask with tinfoil,and the adapter too like a hot air bath. This should help the condensate to travel through to the
condenser. or get some glass wool insulation,just get some from your roof. The oil bath should be sufficient to do the job. I don't know what you mean
by pressurized though as you need to have an outlet fir the pressure or it will break and hot acid is no fun to clean plus a fire will result on
contact with the oil, this is why I use asand bath for this procedure. Just watch for bumping though.
i seem to have thought for some reason you are using a proper distillation setup, but looking at it you are just using a retort right? you can still
make the boiling part insulated with foil but to help the nitric condense along the outlet you can improvise a cooling system with a small water pump
and some tubing to wrap around the stem of the retort, run iced water through it starting the water to run from the end of the stem going up to the
flask so it does not get too hot to be useful. it should work ok. small water pumps can be had for under 10$ off our friend ebay. or better still just
buy a proper distillation setup, they really are worth it if you intend on making alot of nitric acid in the future. also you can make alot in one go
rather than constantly needing to change chemicals in a retort and the leftover potassium sulfate sux to remove too.
[Edited on 21-4-2014 by NeonPulse] |
|
|
|
| Pages:
1
..
6
7
8
9
10
..
80 |