madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
Reduction of carbonyl groups
Will reduction of a carbonyl group with Al/Hg amalgam yield an alcohol, or will the reduction go all the way to hydride? I'm worried it will go
all the way to the hydride, because that's what happens with Zn/Hg amalgam in HCl.
I weep at the sight of flaming acetic anhydride.
|
|
|
Organikum
resurrected
    
Posts: 2337
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
I don´t think this can be answered in an universal valid way. And so it´s down to a: "depends on"
- compound
- where the cabonyl is attached, steric hinderances?
- reaction conditions
- weather in shanghai 27.11.1903 03:15 pm.
alternative I had a strong "maybe" to offer or a somehow instable "perhaps".
More input would be helpful.
ORG
|
|
|
madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
The substrate for reduction would be 1-phenylpropan-1-one.
I weep at the sight of flaming acetic anhydride.
|
|
|
Organikum
resurrected
    
Posts: 2337
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
Propiophenone?
You aim for PPA I guess and some oxazoline from that. I have the patents and refs somewhere - will have a look.
If PPA is the first target you might be interested in the b-dehyde + bakers yeast -> L-PAC reaction. L-PAC is quite easily aminated to PPA by using
ethylamine or NH3 in a reductive amination.
But perhaps my black soul is misguiding my imagination, so tell if it´s on PPA as guessed before I start to look. 
I think the Al amalgan won´t hurt the double bond on the alpha carbon at all in a reductive amination but now I´m on thin ice - better I assure
myself before talking nonsense.
oRG
[Edited on 16-4-2003 by Organikum]
|
|
|
madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
I'm not sure what those acronyms stand for. 
I'm wanting to dehydrate 1-phenylpropan-1-ol by heating with NaHSO<sub>4</sub> to yield propenylbenzene.
I weep at the sight of flaming acetic anhydride.
|
|
|
Organikum
resurrected
    
Posts: 2337
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
Yes this route to the oxazoline is known to me too 
propenylbenzene = allylbenzene is this right? (I hope, MERCK doesn´t help, chemistry is babylon...)
No thats wrong:
from the left to the right, allylbenzene and propenylbenzene and below 1-phenylpropan-1-one aka propiophenone and 1-phenylpropan-1-ol.
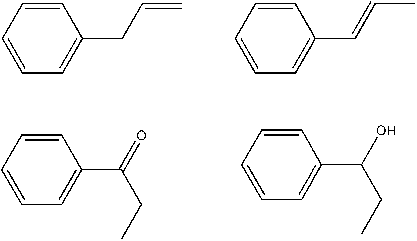
thats better - if chemdraw is right. Is it?
Propenylbenzene won´t be the final product so it´s hard to say if this is the best way. I looked something up and found out that ZnCl2 and HCl would
bear the risk of complete reduction (by chlorination and folllowing reduction of the halogenated compound. As told it is to doubt if aluminium amalgan
whats a complete otherwise working catalyst would even be able to produce the alcohol. The hydrxylgroup woldn´t be touched by the Al/Hg, thats sure.
[Edited on 16-4-2003 by Organikum]
|
|
|
madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
The molecules you diagrammed are the ones I had in mind.
I actually was shooting for a different end result, the next step from propenylbenzene being treating with an organic peracid. Care to explain the
route to the oxazoline propenylbenzene? I'm interested. 
How does Zn/Hg in HCl reduction of a carbonyl proceed? Is an alcohol an intermediate in the reduction? I'm wondering if yields in a reduction of
1-phenylpropan-1-one to 1-phenylpropan-1-ol would be seriously lowered by reduction of 1-phenylpropan-1-ol.
Perhaps this calls for some trials with Al/Hg amalgam reduction of acetone. 
Edit: It just occured to me that the role of the HCl in Zn/Hg reduction of a carbonyl group might be to protonate alcohol intermediate, leaving a
carbocation which might somehow be reduced all the way to methylene with chloride ion involvement (sorry for vagueness; I'm mentally fatigued, so
I can't think it through too clearly)! This means that reduction to alcohol might be accomplished if alkaline conditions are maintained.
[Edited on 16-4-2003 by madscientist]
I weep at the sight of flaming acetic anhydride.
|
|
|
Organikum
resurrected
    
Posts: 2337
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
The Zn/Hg in HCl is the Clemmensen reduction. Messy and low yielding I remember. The best catalyst for this would be NaBH4 - noble metal catalysts
work too is understood, so if you want to sacrifice the pound of palladium you have laying around.....
Al/Hg in acidic enviroment? Wasn´t this a base catalysed reaction scheme at least in reductive alkylations and aminations?
Have to dive in the abyss of my harddisk and memories dungeon.

oRG
found: Ni base catalysts (Raney, perhaps Urishibara) CTH/microwave or pressure will do such things with good yields. Base as promotor is required
(often trimethylamine is used).
Just a quickie.
ATTENTIONE dear Madscientist!
You may call yourself lucky for I stumbled over this:
phenyl-1-propanone -> phenyl-1-propanol catalyzed by Na2S2O4 (sodium dithionite, OTC for removing dye from fabrics) is told to work with good
yields. Sounds like a real winner.....
[Edited on 16-4-2003 by Organikum]
|
|
|
Krypton
Hazard to Self
 
Posts: 90
Registered: 21-11-2002
Location: Spain
Member Is Offline
Mood: explosive 21
|
|
Organikum,
have you a idea to oxidizing of
4-hydroxyimino-2-oxazoline
(-N=CH-O-CH2-C)=NOH
?????
In convention with my ancestor.
|
|
|
Organikum
resurrected
    
Posts: 2337
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
Oxidizing to what?
Naming the target compound would help a lot.
|
|
|
madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
I'm guessing he was wondering how to prepare an oxazoline from propenylbenzene.
How about using some sort of crossed Tishchenko reaction to reduce the carbonyl to an alcohol? As in, preparing a cold solution of propiophenone and
aluminum ethylate in ethanol, then slowly adding, say, acetaldehyde to the solution. Would that yield the acetate ester of 1-phenylpropan-1-ol?
I weep at the sight of flaming acetic anhydride.
|
|
|
Organikum
resurrected
    
Posts: 2337
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
propenylbenzene -> PPA -> 4-MAR
To your question Madscientist: I have no idea if this works and to be true I have problems to imagine the reaction so "free hand" from the
writing. I will (ab)use some imagination enhancers (ChemDraw Ultra and name reaction database) - but this may take some time, sorry.
I still prefer to discuss on at least ONE fixed point - most beloved is still the target compound.
Which one are we after by now? 
lil´confussed
oRG
[Edited on 22-4-2003 by Organikum]
|
|
|
Krypton
Hazard to Self
 
Posts: 90
Registered: 21-11-2002
Location: Spain
Member Is Offline
Mood: explosive 21
|
|
I was wondering because the usage of oxazoline as
a cleaning agent or solvent(?).
I think when oxidized in two or tree steps to
4-nitrimino-2-oxazoline
or from other starting material the way to
2-amino-4-nitrimino-2-oxazoline to
2-nitro-4-nitrimino-2-oxazoline
In convention with my ancestor.
|
|
|
Organikum
resurrected
    
Posts: 2337
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
oxazoline is a class of compounds
And these are depressants mostly. A CAS number would be helpful for the starting compound which to oxidize. (the cleaning stuff)
(btw. I get zero hits for your target compounds on Google andMerck. Chemdraw says it can´t construct a molecule after this name. I am left quite
helpless. Either this is imposssible or the nomenclatura is not perfect IUPAC maybe?)
  
|
|
|
Krypton
Hazard to Self
 
Posts: 90
Registered: 21-11-2002
Location: Spain
Member Is Offline
Mood: explosive 21
|
|
Can you found the nomenclatura of
this somewhere else ?
Apparent, you`re a patent hunter. 
examble:
You can say pentaza, or pentaaza ...
Organikum,
you can look for the CAS number by the DEA.  
In convention with my ancestor.
|
|
|
cha0sad
Harmless

Posts: 1
Registered: 9-10-2003
Member Is Offline
Mood: No Mood
|
|
ppa
orgy,
i aim for ppa from l-pac..
would love it if you could give me more info on that.
end result i want is 4-mar.
swim is VERY thankful to you for your detailed writeups on production of l-pac elsewhere.
swim has had good results using your methods to get to c10h15no.
and of course deoxyephedrine from there.
have a few questions though.
1.swim dont like having hg.or methylamine,
to get to ppa can i aminate with ethylamine or nh3, then reduce?
1a.would activated zn/amonium formate work??
1b.if yes, can i do a reductive amination?(i.e. both at once).if no what % nh3 should be used and what conditions and time?
swim has never done an amination without the redudtion =)
2.would racemazation(sp) be a problem when target is ppa since its (+,-) anyway?
3.do you think l-pac is better way to go than benzyldehyde/nitroethane route?
and again thank you, this bee truly appreciates the help your writeups have been.
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
it'd be nice to get an answer
But Orgy vanished from here and That Other Place near the end of July. I hope he's okay, wherever he may be.
|
|
|
Organikum
resurrected
    
Posts: 2337
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
| Quote: |
orgy, i aim for ppa from l-pac..
|
Norephedrine form l-PAC:
Pressurerized Al/Hg as on Rhodiums page but with ammomia in ethanol (94% alc. is ok). Yield: 50% to 60%.
Add the amine in alcohol to the ketone and let sit for some hours. This should warm up on addition. Then do the Al/Hg under pressure (3 to 5 atm is
ok).
Ratios amine to ketone 1 to 1,5 molar.
your milage may vary
/ORG
|
|
|