| Pages:
1
2
3 |
kABOOM!
Harmless

Posts: 40
Registered: 19-12-2005
Location: Pacific side of Canada
Member Is Offline
Mood: No Mood
|
|
dimanganese heptaoxide based explosives
I was recently experimenting with Potassium Permangante and pure Sulfuric Acid. The idea was to isolate green-oily dimanganese heptaoxide and so far I
have been able to isolate 50 mL of dimanganese heptaoxide. Its pretty violent stuff!
I'm curious if one can make an energetic type of flash powder with this stuff? I know that my dimanganese heptaoxide will ignite rubbing alcohol upon
contact with it!
Anyone else try messing with dimanganese heptaoxide? What could the uses be? Isn't it one of the most violent oxidizers known?
play safe, play hard...get your lumps...pick yourself up and try again. It will only make you stronger.
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
As it is a liquid, it is not possible to make a flash powder with it. It is also explosive by itself. With xylene it detonates. I have always
wanted to try mixing it with Al powder for a thermite type mix, but it might spontaneously combust that way. If you try that, go with very small
ammounts. I was never able to try it due to limited permangante resources. So you isolated 50 mL of it pure? not just the solution of it in sulfuric
acid?
It is some pretty nasty/fun stuff, so be carefull.
|
|
|
kABOOM!
Harmless

Posts: 40
Registered: 19-12-2005
Location: Pacific side of Canada
Member Is Offline
Mood: No Mood
|
|
dimanganese heptaoxide was isolated through using pure, distilled water. I did not want the reaction runaway, so I added Potassium Permangante to the
cold Sulfuric acid. It worked like a charm. Naturally more liquid than solid... I've seen what happens to Potassium Permangante when a few drops of
sulfuric acid are added... very exothermic reaction takes place.
The dimanganese heptaoxide (from now on lets call it Permanganic Anhydride) sat at the bottom of the flask- dark green, and oily. I was able to suck
up a few large globs of the target substance using a glass eye dropper. I then added the target material into some distilled water inside a 100 ml
pyrex flask, then swirling it around to clear the oily product of impurities, I then sucked up the globs again and put the oily liquid into a pyrex
test tube.
Its pretty dense stuff... it will ignite on contact with Methanol, Ethanol and Isopropanol. This must be the stuff... right?
Xylene and Permanganic Anhydride sounds like a BLAST.
[Edited on 27-3-2006 by kABOOM!]
[Edited on 27-3-2006 by kABOOM!]
play safe, play hard...get your lumps...pick yourself up and try again. It will only make you stronger.
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
| Quote: | Originally posted by kABOOM!
I then added the target material into some distilled water inside a 100 ml pyrex flask, then swirling it around to clear the oily product of
impurities, I then sucked up the globs again and put the oily liquid into a pyrex test tube.
|
Really??!! That worked?? I have not had the chance to use large ammounts of
this stuff, but the small ammounts I added to water hydrolyzed to permanganic acid. I have not had the chance to use large ammounts of
this stuff, but the small ammounts I added to water hydrolyzed to permanganic acid.
Isolated through filtration? Care to elaborate? As everything is liquid I do not see the point of this, morover you used some sort of glass filter
right?
I am not 100% sure what you have is Mn2O7 with all this talk of washing with water and filtering.
EDIT: I made a bit of Mn2O7 and added it to water, it lasted longer than I expected, but still dissolved too fast to make a water wash work.
[Edited on 27-3-2006 by rogue chemist]
|
|
|
kABOOM!
Harmless

Posts: 40
Registered: 19-12-2005
Location: Pacific side of Canada
Member Is Offline
Mood: No Mood
|
|
I'm thinking your right. I have a contaminated solution... it looks like their are some crystals still left in the liquid..and its causing the
material to thicken. No clue. I'm trying again.
I spoke too soon!!
UPDATE: humm, now I am really curious if I have the target substance or not. I know that it is reactive! But is it pure** ?? I guess not. The green
oily liquid is soluable in water... interesting, I used my lab grade permanganate and got a different result. Humm. What is your thought on this? This
green oily liquid material created is acidic...very dense too, but soluable in water.
Maybe a mix of permanganic acid/permanganic anhydride??
[Edited on 27-3-2006 by kABOOM!]
play safe, play hard...get your lumps...pick yourself up and try again. It will only make you stronger.
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Well when you added it to water the water did become purple right? For me the green drop slowly dissolves leaving a purple trail. See I used a really
small ammount, essentially a single drop of it, so if you have several mls, dissolution would have been much slower and less noticeable. Personally I
have found it difficult to get the pure Mn2O7 separate from the solution of it in sulfuric acid. I havent got around to following the method in
Brauer which produces it pure.
Yours at least catches a piece of toilet paper on fire right? Thats my favorite thing to do with it
|
|
|
kABOOM!
Harmless

Posts: 40
Registered: 19-12-2005
Location: Pacific side of Canada
Member Is Offline
Mood: No Mood
|
|
Yup. My green liquid appears to dissolve quite slowly in water, leaving some purple trails as you said it would.
Oh, and Aluminum powder acts explosively with Mn2O7... I just tried it.
play safe, play hard...get your lumps...pick yourself up and try again. It will only make you stronger.
|
|
|
Swany
Hazard to Others
  
Posts: 188
Registered: 11-4-2005
Location: My happy place...
Member Is Offline
Mood: Sanguine
|
|
It also explodes nicely with sulfur. And pretty much anything else that resembles fuel, or organic-ness. Rather amusingly, I was sprayed with some of
the H2SO4 stuff when I dropped a large chunk of the 'sludge' that is initally formed on a piece of wood, attempted to retreat, and was promptly
sprayed with the remains of the sludge. It got all over my face/shirt(made holes in it) and it stung. I took the liberty of diving into a snowbank....
I was wearing eye protection, thank goodness.
All in all, tis fun stuff. I cannot really add anything profound to the discussion.
I did have a 'runaway' once, when I added all of the rest of the KMnO4 (about 10g) and left it sit, then came back and it was on fire, spewing ozone,
and making loads of fluffy MnO2. It was all quite amusing, and it smelt... ozonish. The next reaction utulized an ice bath.
|
|
|
kABOOM!
Harmless

Posts: 40
Registered: 19-12-2005
Location: Pacific side of Canada
Member Is Offline
Mood: No Mood
|
|
Man, I just tried a mix of Aluminum, Iron III oxide and sulfur initated by dropping a few drops of Mn207 onto the powder.... 15 seconds later,
KAAAAFOMPH... an absolutely spectacular incendary mix was born.
 this stuff is EVIL! this stuff is EVIL!
play safe, play hard...get your lumps...pick yourself up and try again. It will only make you stronger.
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
| Quote: | Originally posted by kABOOM!
 this stuff is EVIL! this stuff is EVIL! |
And thats why we love it
Attached is the method to produce it pure.

|
|
|
woelen
Super Administrator
        
Posts: 8011
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
kABOOM, could you please specify precisely what you did to make the Mn2O7? From all your posts I have an idea, but a nice step by step recipe would be
nice. I know the method, posted by rogue chemist, but your method sounds very interesting, because it works with water.
If you provide us with a step by step methof of preparing your compound (whether it is Mn2O7 or not), then I'll try to repeat it (but not with the
large amounts you used, I care too much about my fingers  ) and see whether it
really is Mn2O7. ) and see whether it
really is Mn2O7.
I once made a few drops of this, in a warm and very dusty room. I had the drops on a nice glass plate and what I saw was really wonderful. Every few
seconds I saw little flashes/specks of light at the surface of the drop. These little flashes were due to dust particles, which when they entered the
drop, were oxidized at once, with the production of little flashes of "fire". While I was staring at the wonderful phenomenon it suddenly did BANG
 ! Without any apparent reason it exploded and a cloud of fluffy MnO2 was
produced. ! Without any apparent reason it exploded and a cloud of fluffy MnO2 was
produced.
|
|
|
kABOOM!
Harmless

Posts: 40
Registered: 19-12-2005
Location: Pacific side of Canada
Member Is Offline
Mood: No Mood
|
|
sure, try this.
Take 75 ml of pure H2SO4 and *slowly* add a teaspoon amount for course KMnO4. The reaction takes place almost right away. You should see two layers
form, and you are looking for the dark green coloured liquid. Next take a glass eye dropper and suck up a few droplets of the dark green acidic
solution of Mn207/H2S04- it will be fairly dense. Then carefully* fill a small 200ml flask with cold distilled water and add several of the green
drops to it.
I find that the dense Mn2O7 does NOT dissolve right away...
Then I agitated the green blob at the bottom of the water flask and sucked it up again using the glass eye dropper. Using this method I have
collected, I collected around 50ml of green-oily liquid. I also keep the test tube chilled at -1C... the excess water freezes, while the Mn207 is
much like resin.
However, I like Brauer method too... I'll try that as well.
play safe, play hard...get your lumps...pick yourself up and try again. It will only make you stronger.
|
|
|
woelen
Super Administrator
        
Posts: 8011
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I'll try your method, just out of curiousity. I, however, do not intend to store the liquid. I make a small amount (scaling down by a factor of 20),
do some fun experiments with it, and then it is gone. I simply think it is too dangerous to have this stuff around for any period of time.
|
|
|
kABOOM!
Harmless

Posts: 40
Registered: 19-12-2005
Location: Pacific side of Canada
Member Is Offline
Mood: No Mood
|
|
Very true... I'd say Peroxyacetone is far safer than this stuff.
play safe, play hard...get your lumps...pick yourself up and try again. It will only make you stronger.
|
|
|
woelen
Super Administrator
        
Posts: 8011
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I did an experiment with Mn2O7. I was not brave enough to isolate the material, but I did the experiment with a combustible liquid, which is set on
fire by simply touching it. Quite spectacular.
Also nice to see is the purple vapor of Mn2O7. The experiment nicely demonstrates it. Here follows a link. I added the description to my website:
http://woelen.homescience.net/science/chem/exps/mn2o7/index....
EDIT(woelen): Changed link so that it works again.
[Edited on 8-3-13 by woelen]
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Awesome, Woelen. Very nice photo/video shots!
Could you tell precisely how you made Mn2O7, and how you extracted it? Similar to what Kaboom wrote? Also, if you are up for it, did you test its
properties in the presence of dust, minor organic impurities, and so on? Temperature sensitivity? There should be a large amount of experiments that
could be easily done and recorded... I am sure also that this prep would be (in conjunction with kabooms thoughts/observations) would be worthy of an
entry in the library!
I tried reposting one of your images here (with the green oil, http://woelen.scheikunde.net/science/chem/exps/mn2o7/exp7.jp... )to have a picture forever, but somehow I coulnt get it to work, don't ask me
why.
PS Why the heck does it show 'don\'t ask me why.' - I never told it to do so! I think that's why my image uplink didnt work 
[Edited on 29-3-2006 by chemoleo]
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
DeAdFX
Hazard to Others
  
Posts: 339
Registered: 1-7-2005
Location: Brothel
Member Is Offline
Mood: @%&$ing hardcore baby
|
|
Woelen or Kaboom do you know if Mn2O7 oxidizes liquid ammonia(1 atmosphere -33ish celcius). This could make an interesting novel low temp energetic
material...
|
|
|
kABOOM!
Harmless

Posts: 40
Registered: 19-12-2005
Location: Pacific side of Canada
Member Is Offline
Mood: No Mood
|
|
...not sure, but it might! Try it out and see what happens... hell, its easy enough to make. Just, play safe. 
play safe, play hard...get your lumps...pick yourself up and try again. It will only make you stronger.
|
|
|
woelen
Super Administrator
        
Posts: 8011
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
| Quote: | Originally posted by chemoleo
Could you tell precisely how you made Mn2O7, and how you extracted it? Similar to what Kaboom wrote? Also, if you are up for it, did you test its
properties in the presence of dust, minor organic impurities, and so on? Temperature sensitivity? |
Making it is very simple. Just put KMnO4 in concentrated H2SO4 and the oil separates from the liquid. It takes some time however. With some glass
dropper (very carefully cleaned) you can take away the liquid.
Many years ago, I did the test for its stability in the presence of dust (see a few posts above)  . Well, I can say, it is NOT stable in the presence of dust. It just explodes. . Well, I can say, it is NOT stable in the presence of dust. It just explodes.
I've never done experiments with temperature. The stuff I made is at room temperature. It gives of a very thin purple vapor, which stains the glass
nearby with a purple color (you have seen that on my webpage). This purple stain is produced always, even if no reaction is carried out with the
material.
I personally would not make it in more than tiny quantities, and I certainly would not store it. It is a very nice compound for funny experiments
(purple fire with paper, little explosions, flashing as demonstrated on my page), but no serious things can be done with it. Just too unstable.
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
On your page, you describe Mn<sub>2</sub>O<sub>7</sub> as purple in a thin layer and green in a thick layer. I remember
reading somewhere that this difference was due to the different colors seen from transmission and absorption of light. After all, purple and green are
opposites on the color wheel.
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
The Brauer procedure for making it pure is quite the adventure!
I scaled it down 10 times. Kneeding the pure Mn2O7 oil out of the porous MnO2 is pretty nerve wracking, however after 20 min the oil flowed out well
enough. I collected about half a mL of it in a 10 ml volumetric. Now the fun starts. I poured(pipette would not reach down neck well) a few drops
of it on some kleenex. I was expecting instant flash as usual, but it just made the kleenex green. 10s later 'fwump' which actually did decent
shredding damage to the kleenex. Now as I had poured it, there was a drip of the pure oil on the side of the volumetric, so when I put it down on my
cork covered lab bench, it touched cork and BANG(really loud, like a small ammount of silver fulminate). Luckily the contents of the volumetric did
not detonate as well. Then as I was kind of shaking by this point I used the rest to make a solution of HMnO4.
The attached picture shows how volatile the Mn2O7 is. This is after 13h of sitting in the mortar and pestle.
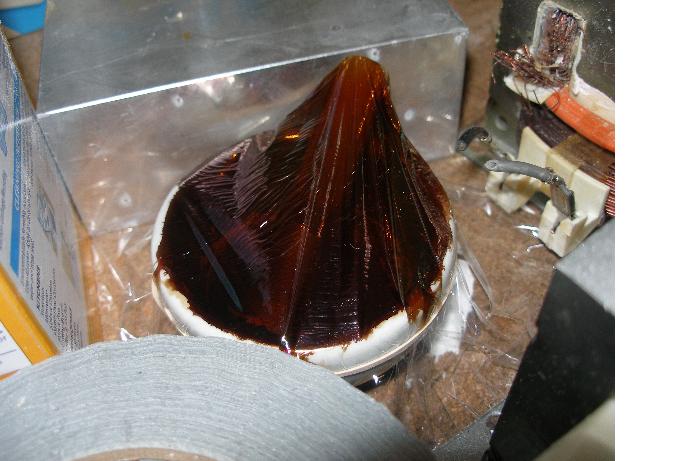
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Wow, very interesting. I've got to try this! Sounds like an instant binary explosive! I seem to remember a video from Axt where he tried xylene and
Mn2O7.
What is the procedure in Brauer, and how is it advantageous to the normal H2SO4/KMnO4 one?
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
I posted the Brauer procedure in an attachment up above in this thread. The advantage is that you produce it pure, free of potassium and sulfate
ions(assuming you do not use too much H2SO4), as opposed to the 'standard' method when you just add KMnO4 to sulfuric acid and pour the resultant
solution on stuff.
It is interesting, the kneeding process that is, as after letting it sit overnight, it looks like you just have MnO2, but when it is carefully scraped
into a corner, the morter angled, and carefully pressed with the pestle, the Mn2O7 flows to the lower part of the pestle. It takes quite some time to
get a good flow as the MnO2 is quite porous, eventually it gleams green with the Mn2O7 oozing from it.
btw, I neglected to clean my mortar and pestle with chromosulfuric acid as the procedure calls for, and I did not suffer disastrous circumstances as a
result.
The vapours of it seem to be effectvly blocked by a dust mask.
EDIT: Any time I say 'flows' above really means slowly oozes.
[Edited on 30-7-2006 by rogue chemist]
|
|
|
agent_entropy
Hazard to Self
 
Posts: 91
Registered: 17-7-2006
Location: U.S.
Member Is Offline
Mood: No Mood
|
|
Awesome, rogue chemist, I was wondering how critical the chromosulfuric acid cleaning was.
Did the procedure have any negative effect on the mortar and pestle? eg. stains, erosion, etc...?
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
There are lots of MnO2 stains, but as evidenced by a quick test earlier from a trial run, it comes off easily enough with HCl. No erosion has been
noticed yet.
Anyway, I took a nap today, and as I was falling asleep I got the idea to try to detonate the Mn2O7/MnO2 mix initially formed. It's not something I
would be able to do where I am, but it could be interesting.
|
|
|
| Pages:
1
2
3 |