Boffis
International Hazard
    
Posts: 1863
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Things to do with Ethyl Formate
Follow Fery’s thread on the preparation of ethyl formate by reactive distillation:
http://www.sciencemadness.org/talk/viewthread.php?tid=155432...
and my own very successful preparations following on from his work, I thought it would be worth looking into the uses of this ester.
The most obvious product from ethyl formate is probably formamide and so experiments were undertaken to investigate the reaction of the ester with
ordinary 28-32% aqueous ammonia. This procedure was described by Phelps and Deming in 1907 1) although this procedure was only seen after my initial
experiments. These experiments were reasonably successful but the degree of vacuum required to distil the product without decomposition could not be
achieved with my water aspirator. Formamide also seem to hang on to the water in it tenaciously. The preparation is easy if you have access to a
mechanical vacuum pump as most textbooks recommend distillation of formamide at 10mm Hg to keep distillation temperature below 100°C (Bp 90° at
10mm). A complex distillation set-up with fraction dividers is probably unnecessary since once the water had been removed the condenser had to be
manually dried or exchanged for a dry one because much condensation hangs in the condenser.
The next preparation I tried was N-methylformamide from ethyl formate and aqueous methylamine. This preparation was likewise successful but the yield
was low due to the loss of product to the middlings during vacuum distillation as this compound has a lower boiling point than formamide and can be
distilled at aspirator pressures. N-Methylformamide can be distilled 103-105° at 75mm Hg without decomposition.
An obvious extension of this type of reaction was the reaction of hydrazine hydrate with ethyl formate. The reaction occurs very readily but the
mixture of reaction products is complex. The nature of the reaction products were investigates by adding salicylaldehyde to the reaction mixture.
Three crystalline products were recovered; almost colourless pearly scales believed to diformylhydrazine, pale yellow plate believed to be
salicylaldehyde formylhydrazone and brilliant yellow prisms of salicylaldehyde-azine. The latter predominated and appear identical to material
prepared from hydrazine hydrate and excess salicylaldehyde (preparation described at the end). The evidence suggests that the reaction is incomplete
using only the spontaneous heating of the reaction mixture but I was reluctant in this preparation to heat the mixture in case this caused the
formation of diformylhydrazine. In the light of my further experiments it appears that this is not a problem and at least an hours refluxing in the
presence of excess ethyl formate is required to consume all of the hydrazine hydrate.
Reactions yet to be tried include various Claisen type reactions such as the formation of the ethyl ester of malonic acid semialdehyde, so called
ethyl formylacetate, and the reaction of a formate ester with acetone to yield 1,3,5-triacetyl benzene mentioned here: http://www.sciencemadness.org/talk/viewthread.php?tid=155719...
Other compounds that can then be produced from those prepared here include diformyl-2,3,5,6-tetrahydroxypiperazine, the precursor to TEX (http://www.sciencemadness.org/talk/viewthread.php?tid=6000#p...), from formamide and glyoxal; Vilsmeier-Haack reagent from methylformamide and
thionyl chloride etc.; formylhydrazones of salicylaldehyde and resorcinaldehyde (reagents of Al, Ga, Be , Zn etc.; 1,2,4-triazole from formylhydrazine
and formamide; 4-amino-1,2,4-triazole from formylhydrazine alone.
A good deal of confirmatory work needs to be done on the products so far. I have yet to find the best solvent system for TLC of the product of the
formylhydrazine reactions. This whole field is very much “work in progress”.
Experimental details
All of these experiments were carried out as preliminary experiments and no photographs were taken.
Formamide
A very small scale experiment carried out with crudely measured quantities in a boiling tube indicated that the reaction is fairly vigorous and mildly
exothermic so a larger scale preparation was carried out.
125g (approx. 1.7 moles) of ethyl formate were placed in a conical flask suspended in a cold water bath on a magnetic stirrer plate. 110ml of 32%
aqueous ammonia (approx. 1.85 moles) were added to the vigorously stirred ester over a few minutes. The mixture became homogeneous almost immediately
and the temperature rose slightly. After 30 minute stirring the flask was removed from the bath and allowed to stand at room temperature for 2 hours.
The reaction mixture was then distilled until the temperature reached 100°. The distillate, comprising water, ethyl alcohol and excess ammonia, was
kept and worked up to recover the ethanol. The residue from the flask was vacuum distilled at aspirator pressure (approx. 90mbars/ 75mm Hg) in a
simple assembly until the still head reached about 90° when distillation stalled. The heating mantle was then removed and a Bunsen burner used to
heat the flask. The flask residue was a clear, very pale straw coloured, slightly oily liquid weighing 94.22g. This is greater than 100% yield (theory
76.0g) so there is clearly still a significant amount of water in the residue.
The residue was vacuum distilled again using a free Bunsen burner flame from the beginning and heating until the still head temperature reached 135°C
(Bp of formamide at 75mm is about 140°C). A small amount of addition distillate boiling between 80 and 135°C was collected but the volume, about
20ml, was too small to be redistilled. The clear pale straw coloured liquid remaining in the still was almost odourless and weighed 73.7g: About 95%
of theory.
Discussion
The purity of the product is uncertain but it is probably adequate for reactions carried out in an aqueous medium. To obtain a pure product a
mechanical vacuum pump will be required to obtain a sufficiently low pressure to ensure distillation without decomposition. Even then the water that
hangs in the condenser means that the product will probably require drying with a zeolite and a second distillation.
N-Methylformamide
60ml of 40% methylamine (c 0.9 moles) solution were added to 50ml (0.62 moles) of vigorously stirred ethyl formate quickly. The mixture became
homogeneous almost immediately and an exothermic reaction caused the temperature to rise to about 45°C. When the exothermic reaction subsided the
flask was placed on a hotplate set at 100° for 30 minutes to complete the reaction. The reaction mixture did not boil in this time.
The solution was left to cool for a couple of hours and then slowly distilled at atmospheric pressure until most of the alcohol and excess methylamine
had distilled off. At about 100° distillation practically ceased. The residue was transferred to a smaller flask and distilled under vacuum at about
70mm Hg. A middle fraction passed over between 40°C (essentially water) to 100°C. The N-methylformamide was collected between 100 and 106°C. It is
a colourless, almost odourless, slightly oily liquid. The yield was 25.59g or about 43% of theory.
Discussion
Considerable product was probably lost to the middlings fraction but this fraction was too small to redistill. The use of a vacuum fractional
distillation set-up and the use of a larger scale may significantly improve yield and reduce water content. The latter is important if the material is
to be used to prepare a Valsmeier-Haack reagent. A much smaller excess of methylamine may be adequate and this would greatly improve the yield
relative to methylamine which is probably the limiting material for most amateurs.
Formylhydrazine
The basic preparation of formylhydrazine comes from the Organic Synthesis preparation of 4-amino-1,2,4-triazole in which it is an intermediate.
37ml (0.75 moles) of 98% hydrazine hydrate were diluted with 50ml of rectified spirit. To this were added slowly and with vigorous stirring over about
5 minutes 55ml (0.68 moles) of ethyl formate. The mixture became homogeneous almost immediately and there was a mildly exothermic reaction. The
mixture was stirred for about 30 minutes and then left to stand overnight.
The reaction mixture was then distilled slowly at atmospheric pressure until the still head temperature had reached about 84°C when most of the
alcohol had distilled off. The liquid was then vacuum distilled using a water pump with gently suction (about 300 mBars) and a further small fraction
of mainly water distilled off at about the same still head temperature. The clear, very pale pink, residual liquid was poured into a glass bowl and
chilled at 4°C overnight. No crystals formed so the liquid was poured into a pre-weighed bottle and re-weighed. 48.48g of product had been recovered
but the material still contains significant water (theoretical yield of anhydrous formylhydrazine is 40.5g).
Discussion
Formylhydrazine is a low melting point solid so it is likely that it failed to solidify due to the presence of water and other compounds. Some
references refer to the tendency of formylhydrazine to decompose into diformylhydrazine and hydrazine on standing so the prolonged distillation to
remove alcohol etc. may not have been a good idea. It may be better to prepare the compound only when required by adding the ester to a slight excess
of well cooled, neat hydrazine hydrate and allowing the mixture to stand for only a few hour before use without isolating the crystalline solid. I
have been unable to locate a preparation for solid formylhydrazine though it can be purchased as such from some chemical suppliers. I have also found
no record of salts of this compound.
Formylhydrazine forms complexes with several metals and it is likely that diformylhydrazine does also though I have not seen any references to such
complexes. A paper by Harries 3) details the preparation and reactions of the lead salt. More important are the highly fluorescent complex formed by
the aromatic aldehyde formylhydrazones with some M3+ cations and Zn2+, Be2+ etc. The most important of these is salicylaldehyde formylhydrazone, a
compound that I was keen to prepare.
Salicylaldehyde Formylhydrazone
5.001g of salicylaldehyde (41 millimoles) were dissolved in 15ml of rectified spirit and 5.00g of crude formylhydrazine solution (the product of the
previous preparation) added with vigorous stirring. The mixture rapidly became slightly warm and a pale sulphur yellow ppt began to form. After 5
minutes the mixture had solidified. A further 10ml of spirit were added and the mixture warmed to try and dissolve the product but to no avail. The
mixture was allowed to cool to room temperature, stirred to disperse and filtered. The cake was washed with a little cold rectified spirit and sucked
dry. After drying at 40°C for several hours the sulphur-like cake weighed 4.914g.
The filtrate was allowed to evaporate slowly over several days in a small beaker. When only about 8-10ml of pale straw coloured liquid remained the
scaly crystals were filtered off, washed with a little alcohol and dried gave 0.285g of very pale straw coloured pearly scales of what are possibly
diformylhydrazine.
1.027g of the crude product was taken from the total yield and an attempt was made to recrystallize it from acetone in which the target compound is
allegedly freely soluble. 10ml of acetone were used to start with but this was clearly nowhere near enough so more acetone was added in units of 5ml
and the suspension heated to boiling before the addition of a further 5ml is a clear solution had not been achieved. In the end 50ml of acetone we
required.
On cooling brilliant yellow bladed crystals formed, they were filtered off, washed with a little acetone and dried to give 0.535g of what appears to
be salicylaldehyde-azine.
The acetone was allowed to evaporate to dryness and the small amount of pale yellow scaly residue leached with 13ml of boiling rectified spirit. The
solution was filtered hot through a small hot Hirsch funnel into a flask standing in hot water at 70°C. On cooling and partial evaporation the
filtrate deposited pale yellow platy crystals; the yield of 0.289g appears to be crude salicylaldehyde formylhydrazone.
The remaining c 3.9g of original product were recrystallized from 200ml of boiling acetone. The solution was allowed to crystallise overnight. The
beautiful brilliant yellow blades were filtered off, washed with a little acetone and dried to give 2.11g of salicylaldehyde-azine.
The filtrate was distilled to recover about 150ml of acetone and the residue poured into a beaker to cool. After about 8 hours some 30ml of liquid
remained and large pale yellow plates and smaller bright yellow blades had formed. These were filtered off and the acetone solution evaporated
further. When almost dry the pale yellow platy crystals filtered off. It appeared to be identical to the earlier product and the two crops were
combined to give 1.775g of impure material. This material was leached repeatedly with 30ml of hot ethanol. Any crystals that formed were filtered off
and the filtrate used to leach the residue again. Three extractions gave 0.873g of crude salicylaldehyde formylhydrazone as pale yellow scaly
crystals. The residue appears to be primarily the azine.
The total crop of crude salicylaldehyde formylhydrazone was 1.160g but it is still visibly contaminated with the azine.
Discussion
The original description of the preparation was not published; it forms part of the dissertation of a Dr. Schöfer at Kiel University in 1892 2). I
have been unable to find a subsequent isolation of the solid formylhydrazine. Its preparation without isolation is described in Organic Synthesis
(Col. Vol. 3, p96) and this was used as the basis of the experiment above. In this article the simple heating of the crude formylhydrazine at about
170° C yields 4-amino-1,2,4-triazole. It appears that this reaction is not unique and the heating of equimolar amounts of formylhydrazine and
formamide yield unsubstituted 1,2,4-triazole: this is another synthesis to try 4).
One doubt I have of my own experiments is the recrystallization of the hydrazines from acetone; itself a ketone and therefore capable of reacting with
any available hydrazine moiety. I wonder; could acetone be capable of displacing the other carboxylic acid or aldehyde groups attached to the
hydrazine derivatives being recrystallized forming say acetone formylhydrazone or an asymmetric azine? I intend to investigate this with TLC.
1) Phelps & Deming; Preparation of Formamide from ethyl formate and ammonium hydroxide; American J. Science; pp173-175; 1907.
2) Some details were published in J. für Prakt. Ch. v51, p180-196 by Schöfer and Schwan but I have only just found this paper.
3) Harries C. D.; Berichte, 1894, p2276-2284; sym-Diethylhydrazine.
4) Pellizzari G.; Gazz. chim. It.; v24, pt2, pp222-229, [1894].
Further Notes
I recently prepared salicylaldehyde azine and attempted to prepare diformylhydrazine for comparison with the products described under the head
“salicylaldehyde formylhydrazone” above. The azine compares well and appears to be identical to the bright yellow crystals recovered from the
attempt to prepare salicylaldehyde formylhydrazone. It was prepared as follows:
5.00g of salicylaldehyde were diluted with 40ml of warm rectified spirit while in a separate beaker 1.039g of 98% hydrazine hydrate were mixed with
10ml of rectified spirit. The two solutions were mixed thoroughly and left to stand. The solution began to turn faintly yellow but little else
appeared to happen, then suddenly the whole solution turned into a thick, creamy-yellow coloured opaque mass. The thick slurry was warmed in a hot
water bath for 15minutes to complete the reaction and then left to cool to room temperature. The thick creamy mass was stirred to disperse it and then
vacuum filtered. The pale creamy yellow cake was washed with about 25ml of rectified spirit which was also used to rinse the beaker. The cake was
dried for 2 hours at 40-45 C to give 4.638g of crude azine. The crude product was recrystallized by adding it to 380ml of boiling acetone, refluxing
until the solid had dissolved, cooling to room temperature and finally chilling in the fridge. The result was 3.785g of brilliant yellow bladed
crystals. The filtrate was distilled to recover roughly 300ml of acetone and cooled to yield a further 0.628g of identical azine. This gives a total
of 4.413g of azine or 92% of theory. The filtrate was discarded.
The “diformylhydrazine” on the other hand was not identical to the product mentioned above. On the other hand the low melting point, about 50 C,
of the product prepared as follows suggests that it is formylhydrazine (Mp 55-58°) rather than the diformylhydrazine (Mp 155-157°):
4.00g of 98% hydrazine hydrate were added to 12.75g of ethyl formate (small excess). The reaction mixture became hot and before it started to cool it
was placed on a hotplate and gently refluxed for 2 hours. The hot solution was poured into a shallow bowl and allowed to evaporate but no crystals
formed. Scratching the bowl and triturating with ether also failed to induce crystallisation. The bowl was placed in the fridge (c 4°) overnight and
the liquid solidified into a mass of moist crystals. The crystals were pressed into a small Hirsch funnel and drained using the vacuum pump and then
washed with ether. When dried at 45° the crystals began to melt locally although not completely, warming above this temperature causes the crystals
to melt easily at temperatures that can be held in the hand. The yield was 3.91g of moist colourless crystals. They are easily soluble in water and
alcohol but insoluble in ether. These properties resemble the mono formylhydrazine.
It is therefore possible that the scaly crystals from the original preparation are the diformylhydrazine; they are clearly not identical.
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
It is impressive that you got such a good purity of formamide after only two distillations.
I've read somewhere it requires 5-6 redistillations to be of acceptable purity(don't ask me where I've seen this), and this resulted in me purchasing
it rather than making it myself.
Didn't wanted to redistill such a simple compound this many times.
What I can recommend from there on, is the preparation of sodium diformylamide from it, if you're interested in, what I would call in an almost
pretentious manner, the "best" gabriel reagent that is out there.
To be truthful, it is not really the absolutely best one, but the best easily prepared and used one without a doubt.
I can recommend experimentation with it, as it surely is a lot of fun and a safe way to make whatever primary amine you're after, in a mild, cheap and
clean manner.
The cleanliness is its biggest advantage, compared to the delepine or many other gabriel reagents, for sure at least of any gabriel reagent else that
I've used.
From primary to secondary halides, it always worked out great and resulted in a pretty clean HCl salt after the hydrolysis 
Also, why isn't this in prepublication where it belongs?
Its a really great post!
|
|
|
Boffis
International Hazard
    
Posts: 1863
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
@karlos; thanks for the reply and comments. I am not sure how pure the formamide is given that I couldn't distil it. I simply had to settle for the
removal of most of the water. If I were going to use it in a water sensetive reaction I would probably dry it with a 3A zeolite.
The sodium diformylamide sounds interesting and I may have a look into this but the use of sodium metal in its preparation suggests that water free
formamide is required; I don't think I am there yet  . I have just used most of
the formamide I prepared in the synthesis of 1,4-diformyl-2,3,5,6-tetrahydroxypiperazine. I am going to try and see if I can aromatize it by oxidation
to a poly hydroxypyrazine. In theory it should be possible to dehydrate the de-formylated piperazine to monohydroxypyrazine too. . I have just used most of
the formamide I prepared in the synthesis of 1,4-diformyl-2,3,5,6-tetrahydroxypiperazine. I am going to try and see if I can aromatize it by oxidation
to a poly hydroxypyrazine. In theory it should be possible to dehydrate the de-formylated piperazine to monohydroxypyrazine too.
I didn't post this in pre-publication because I didn't think it was ready yet. I am still trying to find a way of confirming the identity and purity
of some of the product. I still have to recrystallise the salicylaldehyde formylhydrazone and to confirm that the crystals that resulted from my
attempt to prepare diformylhydrazine are infact the monoformyl derivative.
|
|
|
Boffis
International Hazard
    
Posts: 1863
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
I have been playing around with the synthesis of 1,2,4-triazole from my own formamide and formylhydrazine. After refluxing the two compounds together
and distilling of the volatiles I got a clear thick, high Bp oil but I couldn't get anything out of it. The 1,2,4-triazole is soluble in most solvents
and I didn't fancy trying to vacuum distil it so I tried to precipitate it. I eventually found that it forms a bright sky blue to ultramarine coloured
sparingly soluble complex with copper II sulphate. So my plan now is the precipitate it with this reagent and then decompose it with H2S or something
similar. I did these test-tube scale experiments using 0.1M solutions of various salts and 0.1M sodium picrate but the later did not yield a ppt. Most
of the other metals give soluble complexes. Copper gives a soluble deep blue green solution with the crude triazole oil initially but when I tried to
isolate the complex it rapidly turned into the ultramarine blue insoluble compound.
 The blue insoluble Cu complex The blue insoluble Cu complex
 The Co (orange), Ni (amethyst) and Cu (blue green) soluble complexes. The Co (orange), Ni (amethyst) and Cu (blue green) soluble complexes.
I have now prepared pure salicylaldehyde formylhydrazone and tested it with Al3+ and it yields the required orange fluorescent complex. I tried to
prepare the resorcinylaldehyde formylhydrazone too (reagent for scandium) but the Riemer Tiemann reaction on resorcinol gave very poor results (18%)
and the product is rather brown. I can't remove the brown colour from either the resorcinaldehyde or the final formylhydrazone.
[Edited on 18-12-2020 by Boffis]
|
|
|
Boffis
International Hazard
    
Posts: 1863
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
I have now isolated over 60g of copper triazole sulphate, its composition is as yet unknown but looking at the literature it is probably
Cu(triazole)2SO4 by analogy with the numerous described complexes. Now I want to recover the triazole. I have tried sodium sulphide to precipitate to
copper and then leach the dried residue with isopropanol to remove the triazole. I tried a similar process with hot sodium hydroxide to precipitate
the copper as oxide and then evaporate down to recover the sodium sulphate and triazole and again leach out the triazole with IPA.
Does anyone have any better ideas? I thought about H2S but this would liberate free sulphuric acid which I think will complicate the issue unless
neutralized.
|
|
|
Pumukli
National Hazard
   
Posts: 699
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
Why not combine the two? Alkaline sulfide precipitation, use Na2S!
Then dry it, then extract or distill off the triazole. Or first extract then distill... Seems obvious. Or is it too obvious? Is there a catch?
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2781
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
I would consider reduction -- Mg should recover Cu metal and triazole separately
|
|
|
Boffis
International Hazard
    
Posts: 1863
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Pumukli  | Why not combine the two? Alkaline sulfide precipitation, use Na2S!
Then dry it, then extract or distill off the triazole. Or first extract then distill... Seems obvious. Or is it too obvious? Is there a catch?
|
That's what I did! The problems with the sulphide route is that the copper sulphide is hard to filter out the the yield of triazole is very small
compared to the hydroxide route.
|
|
|
TriiodideFrog
Hazard to Others
  
Posts: 108
Registered: 27-9-2020
Member Is Offline
|
|
Fun fact: Galaxy's centre tastes of raspberries and smells of rum. When astronomers were looking at Sagittarius B2, to find evidence for amino acids,
they did find ethyl formate, the chemical responsible for the flavour of raspberries.
|
|
|
Pumukli
National Hazard
   
Posts: 699
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
Fun fact No 2: those who actually have smelled ethyl-formate are baffled because the smell of ethyl-formate and raspberries are worlds apart!
Admittedly, they shied away from tasting the substance but still. 
|
|
|
Pumukli
National Hazard
   
Posts: 699
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
Filtering CuS: in the old days it was common practice to boil the very fine solid precipitate for a few minutes. Sometimes it yields a much coarser
grained solid and makes filtering much easier. I do remember doing it with CuS, albeit in a test tube and not mixed with triazole.
|
|
|
Fery
International Hazard
    
Posts: 1011
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
reaction of camphor with ethylformate and sodium, I found it in Systematic_Organic_Chemistry_Cumming_Hopper_Wheeler.pdf, preparation 38
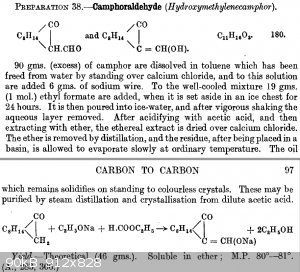
here some info, but almost nothing usefull, this compound seems not to be too much known
https://pubchem.ncbi.nlm.nih.gov/compound/3-_Hydroxymethylen...
|
|
|
Boffis
International Hazard
    
Posts: 1863
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Interesting find, I hadn't thought about Claisen type reactions clearly another one to try. I'm still struggling with isolating 1,2,4-triazole from
the syrup. 
|
|
|
Boffis
International Hazard
    
Posts: 1863
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
I thought this was a good idea so I tried it with magnesium powder on the darker blue 1st crop product in boiling water. After three hours boiling no
reaction had taken place with the copper complex although some white stuff (presumably magnesium hydroxide) has formed but most of the magnesium was
still there.
I worked up the remaining 28g of paler blue 2nd crop of copper complex with sodium hydroxide solution. From my earlier, small scale experiment I was
expecting about 5.4g and I recovered 5.77g of very slightly off white 1,2,4-triazole. I have found that the best method of purification is sublimation
to get pure white prisms of the triazole.
I have not yet found a workable method of decomposing the darker, ultramarine-blue 1st crop, it is usually stable.
|
|
|