woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Getting gas out of N2O capsules in controlled way?
I purchased a few N2O capsules (they only cost me EUR 0.35 per piece, and I have 10 of these things now). These capsules contain 8 gram of N2O. They
look as follows:

The only difference is the brand, I have Kayser capsules, but they look exactly the same. They have a rounded tip, with a circular opening, which is
covered by some metal cap.
How can I get the N2O out of these capsules in a controlled way. Best would be if I could taken some of the gas out of them and keep the rest of the
capsule for further experimenting. One such a capsule has 8 grams of N2O, which is good for 4 liters of gas. Nice for experiments, if I can get it
out in a safe and controlled way.
|
|
|
pantone159
National Hazard
   
Posts: 586
Registered: 27-6-2006
Location: Austin, TX, USA
Member Is Offline
Mood: desperate for shade
|
|
The way that people who want to inhale the stuff extract the gas:
You get some two piece cylindrical device (I think the pieces screw together), and put the capsule inside. One of the ends of of the device has a
hole. Put a balloon on the end with the hole. Screw the device together, this breaks the seal on the capsule, and the balloon fills up.
If you want to breathe the stuff, then you breathe out of the balloon, I forget exactly how you do this. If you could use the filled balloon as a
reservoir, then this might work from you.
Depending on where you bought the capsules, the same place might have these things.
|
|
|
Maja
Hazard to Others
  
Posts: 143
Registered: 27-2-2006
Member Is Offline
Mood: No Mood
|
|
Maybe try using this device :

Are you located in Europe ? Where did you find N2O capsules ? I searched really hard, but without any luck ... I'm from Lithuania.
|
|
|
-jeffB
Hazard to Others
  
Posts: 185
Registered: 6-12-2007
Member Is Offline
Mood: No Mood
|
|
Many years ago, I got a "handheld butane-oxygen torch" from Radio Shack. It takes two cylinders in that same format, one carrying butane, one
carrying N2O. It has thumbwheel controls on the top offering fine control over the flow of each gas. I believe they also sold a single-cylinder
model that burned only butane, but it could probably be used to release N2O in a controlled fashion instead. It looks like Radio Shack no longer
sells them, in keeping with their general trend toward uselessness.
Here's a kit for the two-cylinder torch, at a scandalous price (US$112):
http://www.thomassci.com/catalog/product/775
I've also seen CO2 cylinders in the same format. You might investigate devices that use these cylinders, and see if one can be adapted. Paintball
guns?
|
|
|
msp2
Harmless

Posts: 9
Registered: 10-3-2007
Member Is Offline
Mood: No Mood
|
|
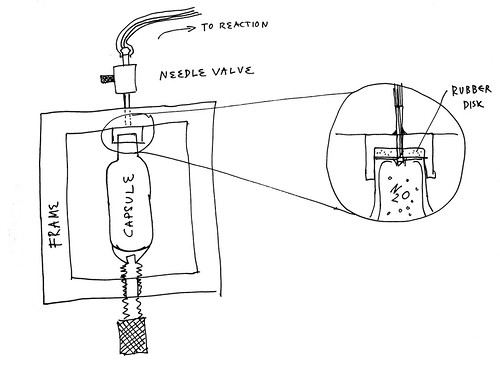
Make the rubber disk thicker than the amount the 'hole punching pipe' extends so that it will seal to the top of the capsule before it is penetrated.
In a CO2 pellet gun I had some years ago, the metal membrane was penetrated by small diameter pipe with rather heavy walls compared to ID, a section
was cut from it to present a sharp edge to allow it to cut through, perhaps it was at an angle.
The torches are usually sold under the name microflame.
Seems ppl have used them before for rocketry.
A better view:

Apparently someone makes oxygen and argon cylinders in aproximately the same size, yay, 4 grams of oxygen.
[Edited on 6-3-2008 by msp2]
|
|
|
Intergalactic_Captain
Hazard to Others
  
Posts: 227
Registered: 4-9-2004
Location: somewhere where i don\'t know where i am
Member Is Offline
Mood: frabjous
|
|
Holy shit, that's the coolest thing I've ever seen msp2 - Any idea where I can get one? I'm guessing I'd have better luck at a hobby shop than a
hardware store, but this seems like one of the things that the American Nanny-State would rather I didn't get my hands on.
If you see me running, try to keep up.
|
|
|
-jeffB
Hazard to Others
  
Posts: 185
Registered: 6-12-2007
Member Is Offline
Mood: No Mood
|
|
That's exactly the one, @msp2. Well, not exactly -- mine has a plastic frame, not metal. I'll try to remember to upload a photo sometime.
Googling for "microflame torch" is a bit more productive. Looks like they're going for US$60 now -- mine was $25 about 30 years ago, which is
probably even more than US2008$60. Here's one link:
http://www.azuremoon.com/tek9.asp?pg=products&specific=j...
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I followed the suggestion of Maja. I have taken a whipped cream dispenser with a 250 ml pressure vessel.
The device works nice and simply. At the top, you put the N2O ampoule and it is screwed into an opening and at a certain point, all gas from the
ampoule is released into the pressure vessel (which normally also contains the cream, but in my case it just contains air). When the gas is in the
pressure vessel, the ampoule can be taken away. The gas now can be slowly released by pressing a handle.
The first time, there still is some air-impurity (appr. 300 ml of air is mixed with 8 grams of N2O). When the gas mix is released and pressure has
dropped too much, then a new ampoule can be emptied in the pressure vessel. So, I filled the pressure vessel with one ampoule, let it all flow away by
pressing the handle, and then the gas from a new ampoule was put in the pressure vessel. In this way, the initial impurity of air is almost gone and
now I have almost pure N2O. The next ampoule it will even be better. You simply never should open the pressure vessel. If you do so, then new air can
get in.
One could also release multiple ampoules into the pressure vessel, but I am not sure how safe that will be. The pressure vessel most likely is not
intended to be used with more than one ampoule, so I did not try that.
Altogether I am quite happy with this. The dispenser cost me appr. EUR 35, including the price of shipping. It allows me to release the gas in a slow
and controlled way by carefully pressing the handle. A thin flexible tube can easily be attached easily to the outlet.
|
|
|
YT2095
International Hazard
    
Posts: 1091
Registered: 31-5-2003
Location: Just left of Europe and down a bit.
Member Is Offline
Mood: within Nominal Parameters
|
|
that little torch looks Very impressive! I think I know what I want for Christmas this year 
up to yet, working with Borosilicate glass (even to flame polish) is next to impossible, something like that would make life Much easier.
Thanks for the info msp2 
\"In a world full of wonders mankind has managed to invent boredom\" - Death
Twinkies don\'t have a shelf life. They have a half-life! -Caine (a friend of mine)
|
|
|
-jeffB
Hazard to Others
  
Posts: 185
Registered: 6-12-2007
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by YT2095
that little torch looks Very impressive! I think I know what I want for Christmas this year 
up to yet, working with Borosilicate glass (even to flame polish) is next to impossible, something like that would make life Much easier.
Thanks for the info msp2  |
Don't get your hopes up too much. The flame is very tiny, too tiny to work borosilicate without setting up excessive thermal stress. I
think I tried to use mine to flame-polish a chipped test tube, and ended up creating a much larger crack.
|
|
|
Broken Gears
Hazard to Self
 
Posts: 96
Registered: 7-8-2005
Location: Northern Europe
Member Is Offline
Mood: No Mood
|
|
I made a fancy little tool for just the job. Back in the days I used to fill ballons with N2O and inhale 
[Edited on 28-3-2008 by Broken Gears]
[Edited on 28-3-2008 by Broken Gears]

|
|
|
Broken Gears
Hazard to Self
 
Posts: 96
Registered: 7-8-2005
Location: Northern Europe
Member Is Offline
Mood: No Mood
|
|
a quick schematic for the top part
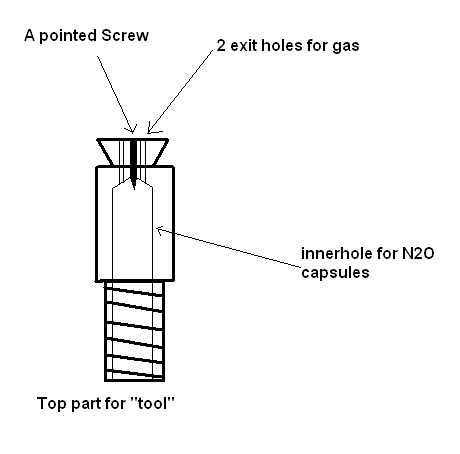
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
The tool is good for filling a balloon from one capsule, but it is hardly useful for controlled release of the gas, because in a balloon it cannot be
stored for long (these are somewhat porous).
The whipped cream dispenser has a heavy pressure chamber and in that the gas can be stored for a long time. So, a punched ampoule with N2O can be used
in small portions and does not need to be consumed at once.
|
|
|
YT2095
International Hazard
    
Posts: 1091
Registered: 31-5-2003
Location: Just left of Europe and down a bit.
Member Is Offline
Mood: within Nominal Parameters
|
|
something else you May be able to get a little cheaper, is a type of Waiters Bottle opener, it`s a CO2 cartridge in a plastic case with a strong thick
needle at one end of the case and a button at the other.
the needle goes through the cork in the bottle, you depress the button and CO2 is delivered down the needle creating over-pressure inside the bottle
and the cork pops out.
you can do about 15 bottles with a single cartridge, I not sure of their Proper name, but something like is ideal for small scale work, I`v made a
variety of carbonates this way myself.
a Specialist Wine or Catering shop may have them.
here, these are the sort: http://www.nextag.com/wine-cork-opener/search-html
[Edited on 29-3-2008 by YT2095]
\"In a world full of wonders mankind has managed to invent boredom\" - Death
Twinkies don\'t have a shelf life. They have a half-life! -Caine (a friend of mine)
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I now can take out the gas slowly and nicely controlled. Of course that needs to be celebrated with a new experiment  (also thanks to ScienceGeek, for the idea behind this experiment through his YouTube
video, search for Mabakken and you easily find all of his good videos). (also thanks to ScienceGeek, for the idea behind this experiment through his YouTube
video, search for Mabakken and you easily find all of his good videos).
This is what N2O can do:
Webpage: http://woelen.homescience.net/science/chem/exps/barkingdog/i...

Edit(woelen): Made link and image work again.
[Edited on 30-7-16 by woelen]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
http://www.electro-sensors.com , Electro-Sensors Inc., 6111 Blue Circle Drive, Minnetonka, Minnesota 55343 , (952) 941-8171 , (952) 945-2800
through it's Microflame Inc. subsidiary, 14873 DeVeau Place, Hopkins, Minnesota 55345 , Phone: (612) 935-3777, Fax: (612) 935-7677
is engaged in the manufacture and distribution of small gas torches and related accessories , The Company's "Microflame" torch is
comprised of
two high pressure cylinders, one containing Butane and the other containing N2O oxidizer, which produces a 5000º F flame with a tip the size of a
pencil point. Microflame sells several kits based upon this basic torch. The kits differ in the number of Micronox replacement
cylinders, brazing rods and other accessories which they contain. This and their Cub butane torch seen here below , which for bleeding a single N2O
cylinder would probably serve you better also used to be available from Radio Shack now discontinued. Click the image to go to the item


This particular kit is now sold for $ 90 U.S., and scarcely available
https://store.sra-solder.com/product.php?xProd=6015
http://www.azuremoon.com/tek9.asp?pg=products&specific=j...
and in Great Britain
http://www.hobby.uk.com/shop/prodpages/page-MI1000.html
http://www.ema-models.com/shop/prodpages/page-MI1000.html
.
|
|
|
ScienceGeek
Hazard to Others
  
Posts: 151
Registered: 22-1-2008
Location: Norway
Member Is Offline
|
|
woelen, I just read through your experiment, and as always: excellent work. It's amazing how you do it, because it just keeps getting
better and better!
Also, thank you for crediting me. I take as a very big compliment that you want to replicate my experiment 
|
|
|
msp2
Harmless

Posts: 9
Registered: 10-3-2007
Member Is Offline
Mood: No Mood
|
|
I got hold of a few capsules today, measured them. May be good to know they are not the same size as the CO2 capsules (so they may not work well with
say bottle openers, etc. without modification)
N20
Lenght: 65 mm
Diameter: 17.9 mm
Neck diameter: 8.5 mm
Fill: ~ 7 grams
CO2
Lenght: 83 mm
Diameter: 18.7 mm
Neck diameter 7.4 mm
Fill: ~ 12 grams
[Edited on 8-4-2008 by msp2]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
This company has just about dropped out of sight , a pity since its
products are unique and have been around for some 40 years.
The "Cub Mini Torch" accepts a single cylinder and appears best suited
to metering gas.
http://72.41.12.125/microflame/torches.cfm
http://72.41.12.125/microflame/microstore/microstore.html
This other webpage only appears sporadically
http://www.azuremoon.com/tek9.asp
http://72.41.12.125/tek9.asp
http://72.41.12.125/tek9.asp?pg=products&grp=11&sort...
" Ordering from AzureMoon is easy! We give you several options. You can browse through our
on-line catalog and choose items as you go along. If you don't want to order on-line you can
fax your order to us at 1-603-471-3845 or call us with your order at 1-877-638-6724
(1-877-MFTORCH)."
Another source perhaps
http://www.mandel.ca/products/chromatography/Gas_Chromatogra...
________________
Ebay has N2O cylinders at affordable cost
http://cgi.ebay.com/ws/eBayISAPI.dll?ViewItem&item=14056...
http://cgi.ebay.com/ws/eBayISAPI.dll?ViewItem&item=13053...
Butane cylinders of this type are practically unknown , does anyone have
a notion of a manufacturer of bottled gas of this type ?
.
|
|
|
Fleaker
International Hazard
    
Posts: 1252
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
Woelen,
Forget using the capsules. I suggest you consider the use of sulfamic acid and nitric acid. Both are quite cheap. Heat concentrated nitric acid with
sulfamic acid and nitrous oxide will be produced. You can scrub any nitrogen oxides and water out of it by running it through a column of NaOH prills.
You can make about 1 kg of N2O for about 10-15 EUR.
Neither flask nor beaker.
"Kid, you don't even know just what you don't know. "
--The Dark Lord Sauron
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Fleaker  | " consider the use of sulfamic acid and nitric acid. "
" You can make about 1 kg of N2O for about 10-15 EUR." |
HSO3NH2 + HNO3 => H2SO4 + H2O + N2O
@ Fleaker , Please show me the math.
I'm all for do it yourself approaches , but your assertion depends on cost
per mole of supplies , and does not consider the convenience of ready made
bottled gas delivered at 52 cents per 8 gram cylinder.
- in this offer ~ 384 grams for 25 dollars US ,
http://cgi.ebay.com/ws/eBayISAPI.dll?ViewItem&item=14056...
$ 25 at 0.7 Euro to the Dollar = € 17.5
http://finance.yahoo.com/q/bc?s=EURUSD=X&t=1y&l=on&a...
.
|
|
|
Fleaker
International Hazard
    
Posts: 1252
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
That's a rough approximation I suppose and more tuned to my thought of the economy of scale; at the amateur level, you may be right.
Here's how I figured it:
67% HNO3 is $0.31/454 g, so $ 0.68 / kg at say 16 M (~ 67% w/v). It is 63.01 g/mol. This means 670 g of nitric acid per kg or 10.6 moles per kg.
99% HSO3NH2 is $0.65/454 g, so $1.43 / kg. It is 97.09 g/mol or 10.3 mol to the kg. An excess of sulfamic is indicated, so 11.3 mol to 10.6 moles (or
1 kg of nitric acid).
Let's say this gives you 10 moles of nitrous oxide (even though it is quantitative, being driven by the thermodynamics of forming a gas that leaves
the solution). At 46 g/mol for N2O, a kg of 67% nitric acid ($0.68) and say 1.1 kg of sulfamic acid ($1.58), so $2.30 or so nets you 460 g of crude
N2O; this puts you at about $5 dollars per kg without the cost of NaOH to scrub any residual nitrogen oxides and moisture, let alone activated carbon.
Lye is cheaper than nitric acid, and activated carbon of sufficient quality, about the price of sulfamic. One must also consider the cost to heat and
the equipment.
The price for sulfamic is based on a 50# sack from Univar, as well as a 55 gallon SS 316 drum of HNO3.
I've used this reaction many times to rid solutions of nitrogen oxides so that I can precipitate various precious metals and/or remove lead. Sulfamic
is a great chemical for removing nitric acid from hot solutions to aid in precipitation of gold, platinum, (and to a lesser extent, Pd and Rh as
they're both soluble in the sulfuric acid!)
Neither flask nor beaker.
"Kid, you don't even know just what you don't know. "
--The Dark Lord Sauron
|
|
|
Texium
|
Thread Moved
19-11-2023 at 16:39 |