slightlymadscience
Harmless

Posts: 4
Registered: 31-3-2008
Member Is Offline
Mood: Inquisitive
|
|
Advice on scaled-up H20 Electrolysis?
I've done small-scale H20 and NaOH electrolysis with test tube/beaker (crucible with NaOH) set ups using DC batteries and carbon electrodes. My
question comes up after scaling up considerably.
Useful info:
I built a plexiglass tank (holds at least 5 gallons) that has gas traps on either side (with valves), and the electrodes are an array of 11 stainless
steel plates (switch plate covers) on either side. The electrodes are separated by a distance of over 30 cm. The power supply is an old solid-state
car battery charger (12 volts, settings of 2/5/10 amps). I've used tap water, and distilled water, and have tried NaCl and NaHCO3 (table salt and
baking soda) as electrolytes.

I based my design on a project done by someone else, but they wanted didn't need to separate the Oxygen and Hydrogen, so their array was a series of
alternating anode/cathode plates that were close together. His reaction was VERY energetic, and produced a lot of gas quickly.
His electrode array (1): +-+-+-+-+-
Mine (2): ++++++ -------
When applying the current, bubbles form on both electrodes, but VERY slowly. I'm wondering if the difference in effect is due to the distance the
current must travel between anode and cathode. I haven't found any resources that could verify this, however.
Any insight would be appreciated.
Thanks in advance.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
That huge gap in the center is killing you. Most electrolytes are not that conductive, so there is considerable resistance in the region between
electrodes; I'd bet you can measure a voltage across that open region by sticking voltmeter probes on either side.
The back side of electrodes doesn't contribute as much to current flow as the front, and electrodes hidden behind others are even less effective - the
front electrode shields them to an extnt.
So remake the tank with very little gap between the two chambers, the cathode and anode should be as close together as practical. I'd use a single
separator, have the solid section extended to below the electrodes using a porous membrane - non-woven plastic fabric works well. Rotate the
electrode arrays 90 degrees so their plates parallel the sides of the tank; better yet make new ones that are much larger than these, reaching nearly
to the bottom of the tank. Use an area behind one or both electrodes to proved access for filling the tank, instead of between them.
With ferrous alloy electrodes stick with alkaline electrolytes and avoid chlorides and other halides like the plague. Hydroxide or carbonate is
better than bicarbonate.
|
|
|
microcosmicus
Hazard to Others
  
Posts: 287
Registered: 31-12-2007
Member Is Offline
Mood: spin up
|
|
| Quote: |
I based my design on a project done by someone else, but they wanted didn't need
to separate the Oxygen and Hydrogen,
|
Not separating the H2 from the O2 sounds like an AWFUL
idea to me, especially on a large scale, because the mixture is
explosively flammable, so I'm glad to see you're not trying that.
| Quote: |
I'm wondering if the difference in effect is due to the distance the current must travel between anode and cathode. I haven't found any resources that
could verify this, however.
|
It's just good old Ohm's law ---- the further apart the electrodes,
the greater the resistance, so the less the current and the less
gas produced. So I would suggest measuring the resistance
of your cell or the current while electrolyzing. If the current is
all that your power supply can put out, then placing the electrodes
closer together won't do much except possibly burn out your
power supply if it does not limit the current it produces. If the
current is well below what your power supply can put out, only then
consider bringing the plates closer to decrease resistance.
| Quote: |
When applying the current, bubbles form on both electrodes, but VERY slowly.
|
As for how fast the bubbles form, remember that a mole of
electrons is 26.8 amp-hours. Running at the full 10 amps your
charger is capable of will therefore produce hydrogen at the rate
of 373 mg/hr. At STP, that amounts to about a gallon of H2 in an
hour. The bubbles may be coming slowly simply because
that is all your power supply is capable of.
[Edited on 1-4-2008 by microcosmicus]
|
|
|
slightlymadscience
Harmless

Posts: 4
Registered: 31-3-2008
Member Is Offline
Mood: Inquisitive
|
|
| Quote: | Originally posted by not_important
That huge gap in the center is killing you. Most electrolytes are not that conductive, so there is considerable resistance in the region between
electrodes; I'd bet you can measure a voltage across that open region by sticking voltmeter probes on either side.
The back side of electrodes doesn't contribute as much to current flow as the front, and electrodes hidden behind others are even less effective - the
front electrode shields them to an extnt.
So remake the tank with very little gap between the two chambers, the cathode and anode should be as close together as practical. I'd use a single
separator, have the solid section extended to below the electrodes using a porous membrane - non-woven plastic fabric works well. Rotate the
electrode arrays 90 degrees so their plates parallel the sides of the tank; better yet make new ones that are much larger than these, reaching nearly
to the bottom of the tank. Use an area behind one or both electrodes to proved access for filling the tank, instead of between them.
With ferrous alloy electrodes stick with alkaline electrolytes and avoid chlorides and other halides like the plague. Hydroxide or carbonate is
better than bicarbonate. |
Thank you for the reply! Since it's the gap that's killing me (which seemed to be the most likely culprit from my perspective), it's no wonder that
the other's rig was so much more energetic - he had alternating anode and cathode plates very close together. I had also thought that the arrays
would increase the reaction due to increased surface area, but since the current doesn't have a direct path (i.e. the facing portion of the electrodes
are most important, like you said), it doesn't really help.
When I get to revisit this, I'll keep your advice in mind.
|
|
|
slightlymadscience
Harmless

Posts: 4
Registered: 31-3-2008
Member Is Offline
Mood: Inquisitive
|
|
| Quote: | Originally posted by microcosmicus
Not separating the H2 from the O2 sounds like an AWFUL
idea to me, especially on a large scale, because the mixture is
explosively flammable, so I'm glad to see you're not trying that.
|
Probably for the best that you don't look at my videos/website. I tend to deliberately do a lot of ill-advised things. 
| Quote: | Originally posted by microcosmicus
the greater the resistance, so the less the current and the less
gas produced. So I would suggest measuring the resistance
of your cell or the current while electrolyzing. If the current is
all that your power supply can put out, then placing the electrodes
closer together won't do much except possibly burn out your
power supply if it does not limit the current it produces. If the
current is well below what your power supply can put out, only then
consider bringing the plates closer to decrease resistance.
|
One lesson I learned with this adventure is to try smaller scale versions or cheaper prototypes before I invest in a whole bunch of plexiglass and
spend a bunch of time making a rig I'm not confident will work. It helped me with future demonstrations, but made me generally discouraged towards
that experiment (I stoppped making videos for over 6-7 months)... I'm glad to get some good feedback this time around. Thanks.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Tip: look at your cell from the standpoint of gaussian surfaces. The electric field created by each electrode is almost indistinguishable from a
square box of the same overall dimensions. Your attempt at high surface area is in fact useless.
You really need interleaved electrodes.
Tim
|
|
|
Twospoons
International Hazard
    
Posts: 1282
Registered: 26-7-2004
Location: Middle Earth
Member Is Offline
Mood: A trace of hope...
|
|
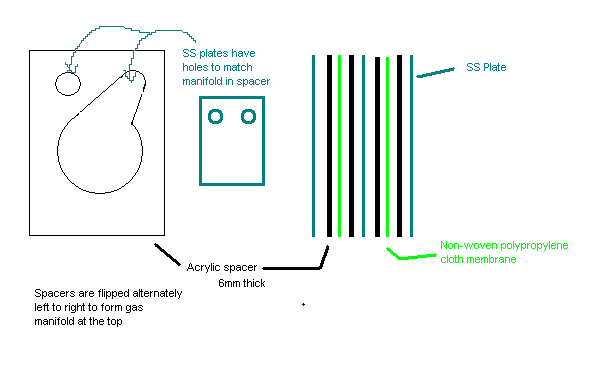
Here is a crude sketch of the electrolyser I built from stainless steel, 6mm acrylic, and non-woven polypropylene cloth. The sketch shows two series
stacked cells - the one I built has 4 cells. The cunning thing about this design is that all the acrylic spacers are identical, but alternately
flipped in assembly, creating a gas manifold which keeps the H an O separate.
The whole thing was stacked and glued with epoxy, and a couple of PVC pipe elbows attached to the gas manifold.
I found the best electrolyte to be NaOH. In my 4 cell stack, supplying 15A at around 20V there was sufficient H2 coming off to sustain a flame.
I should add that this setup really needs some sort of cooling loop for long term operation. Electrolysis isn't particularly efficient.
[Edited on 2-4-2008 by Twospoons]
[Edited on 2-4-2008 by Twospoons]
Helicopter: "helico" -> spiral, "pter" -> with wings
|
|
|
Twospoons
International Hazard
    
Posts: 1282
Registered: 26-7-2004
Location: Middle Earth
Member Is Offline
Mood: A trace of hope...
|
|
Putting my electrolyser to good use
Here's a short vid of what happens when you ignite a foam of mixed hydrogen and oxygen (yes - inspired by "myth busters"). You could feel the
shockwave slap you in the chest. Hearing protection is essential. Damn, that was a fun night.
Attachment: Hydrogen foam.wmv (951kB)
This file has been downloaded 1082 times
Helicopter: "helico" -> spiral, "pter" -> with wings
|
|
|
ScienceGeek
Hazard to Others
  
Posts: 151
Registered: 22-1-2008
Location: Norway
Member Is Offline
|
|
hehe...great video twospoons!  A lot of energy in those two little diatomic
molecules. A lot of energy in those two little diatomic
molecules.
I'm telling ya: not too many years from now, every car will run on water.
|
|
|
roamingnome
Hazard to Others
  
Posts: 363
Registered: 9-9-2006
Member Is Offline
Mood: No Mood
|
|
Your apparatus work is looking good..
a few design quirks to tweek.
we all have old projects laying around that had issues
the PVC tube that encased this experiment melted and bent.
this suker would freely draw 100 amps
and as in twospoons video it could produce a head snapping boom
the classic issues with water electrolysis still defeat us.
so i put the electrodes close together but the gases mix
you put the electrodes far away but current draw is an issue
buying PEM's pricey
im thinking there is a palladium membrane that will let the H2 diffuse through, but block O2, this might be viable
[Edited on 2-4-2008 by roamingnome]

|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
A palladium membrane would likely catalyse the reaction between oxygen and hydrogen - kaboom. There are membranes that would do the job but they're
not that easy to come by.
Twospoons has a good solution, not only do you get the electrodes close together but you can use both sides of them as well.
NaOH is the best electrolyte, but it will absorb CO2 from the air unless protected from prolonged exposure. Sodium carbonate already has absorbed the
CO2 :-) and is cheap and easy to store - your choice.
|
|
|
Twospoons
International Hazard
    
Posts: 1282
Registered: 26-7-2004
Location: Middle Earth
Member Is Offline
Mood: A trace of hope...
|
|
The NaOH does have one other drawback - a tendency to produce foam. (thats not what the foam in the video is - thats detergent). Some kind of foam
breaker is on my 'to do' list for the electrolyser.
| Quote: | Originally posted by not_important
not only do you get the electrodes close together but you can use both sides of them as well.
|
Efficient material use was one of my goals with this design. Stainless and acrylic are not especially cheap materials.
Helicopter: "helico" -> spiral, "pter" -> with wings
|
|
|
slightlymadscience
Harmless

Posts: 4
Registered: 31-3-2008
Member Is Offline
Mood: Inquisitive
|
|
| Quote: | Originally posted by Twospoons
Efficient material use was one of my goals with this design. Stainless and acrylic are not especially cheap materials. |
True, but at least they're readily available. It's nice when you can get most of your materials at the local hardware store. 
I really appreciate all of the great replies to this. I'll definitely be revisiting this once I get some other videos (like playing with powdered
magnesium) out of my queue.
|
|
|