nightshade
Harmless

Posts: 26
Registered: 14-11-2004
Member Is Offline
Mood: No Mood
|
|
what is it?
No one as far as I know has done a reduction of phenylalalnine,so I did but really was expecting any thing.
Been looking in some books still at alost as to what it is.
Changing phenylalnine to blank is like the alchemist dream.
|
|
|
evil_lurker
National Hazard
   
Posts: 767
Registered: 12-3-2005
Location: United States of Elbonia
Member Is Offline
Mood: On the wagon again.
|
|
There are some compounds that you just can't do much with no matter what you throw at them due to whatever.
This is one of those where you simply can't go from point "a to point "b".
I think it remains best to continue to dream on this subject, preferably elsewhere before you attact the attention of the "powers that be", if you
have not already.
[Edited on 7-5-2008 by evil_lurker]
Not all chemicals are bad. Without chemicals such as hydrogen and oxygen, for example, there would be no way to make water, a vital ingredient in
beer.
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
It should be able to be accomplished by a method of reduction of carboxylic acids, that would not affect the alpha-amino group. LiAlH4 in ether
reduces carboxylic acids and esters to alcohols (but not further, apparently), which cannot be further reduced directly. Catalytic hydrogenation may
also hydrogenate the benzene ring. To reduce the R-OH to R-H looks as if it would involve reaction with a halo-acid HX (preferably HI, followed by
HBr) to form R-X, which could be reacted with something like LiH or NaH or MgH2 to remove the halogen as a metal halide. BUT, because the HX would
also and preferentially form an amine salt with the alpha -NH2 group, either an excess of HX would be needed, or that -NH2 group would have to be
protected somehow.
[Edited on 8-5-08 by JohnWW]
|
|
|
azo
Hazard to Others
  
Posts: 163
Registered: 12-2-2008
Member Is Offline
Mood: No Mood
|
|
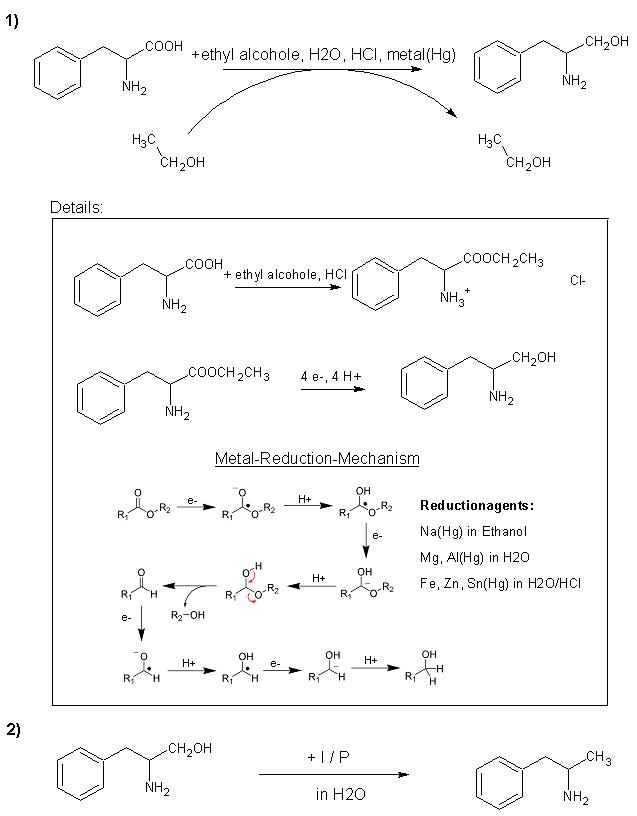
As Johnww said you can do this but it is difficult the only way that i no of is via the halide using thionyl chloride ! Or you could reduce the
carbonyl group with sodium borahydride or another metal hydride reducing agent and then reduce the diol with red phosphorus and Iodine or
Hypophosphorus acid and Iodine the best way is the later.
The best way is proberly not to try at all it will definently bring you trouble at some time.
regards azo.
|
|
|
Bolt
Hazard to Others
  
Posts: 188
Registered: 26-3-2007
Member Is Offline
Mood: No Mood
|
|
I don't know whether or not this will work, but a guy at another forum thought it would.
|
|
|